Emulsion Surimi Gel with Tunable Gel Properties and Improved Thermal Stability by Modulating Oil Types and Emulsification Degree
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fatty Acid Composition
2.3. Extraction of Myofibrillar Proteins
2.4. Preparation of Pre-Emulsified Oils
2.5. Droplet Diameter
2.6. Particle Size and Distribution
2.7. Emulsifying Activity (EAI) and the Emulsifying Stability Index (ESI)
2.8. Preparation of Emulsion Surimi Gel
2.9. Color
2.10. Gel Strength
2.11. Water Holding Capacity
2.12. Texture Analysis
2.13. Rheological Properties
2.14. Determination of Protein Solubility
- index of ionic bands = SB − SA;
- index of hydrogen bonds = SC − SB;
- index of hydrophobic interactions = SD − SC;
- index of disulfide bond = SE − SD.
2.15. Statistics Analysis
3. Results and Discussion
3.1. Fatty Acid Composition
3.2. Droplet Size and Distribution
3.3. Emulsifying Properties
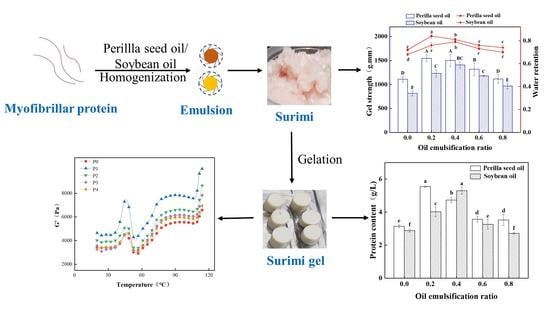
3.4. Gel Properties
3.5. Rheological Properties
3.6. Protein Solubility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wijayanti, I.; Singh, A.; Benjakul, S.; Sookchoo, P. Textural, sensory, and chemical characteristic of threadfin bream (Nemipterus sp.) surimi gel fortified with bio-calcium from bone of asian sea bass (Lates calcarifer). Foods 2021, 10, 976. [Google Scholar] [CrossRef]
- Zhang, L.L.; Yong, X.; Jie, X. Effects of high-temperature treatment (≥100 °C) on Alaska Pollock (Theragra chalcogramma) surimi gels. J. Food Eng. 2013, 115, 115–120. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Pan, D. Gel-forming ability of surimi from grass carp (Ctenopharyngodon idellus): Influence of heat treatment and soy protein isolate. J. Sci. Food Agric. 2006, 86, 5. [Google Scholar] [CrossRef]
- Gao, X.Q.; Xiong, G.Y.; Fu, L. Water distribution of raw and heatinduced gelation of minced pork paste prepared by soy protein isolates and carrageenan: Ingredients modify the gelation of minced pork. J. Food Process. Preserv. 2019, 43, 12. [Google Scholar] [CrossRef]
- Benjakul, S.; Wonnop, V.; Chakkawat, C. Effect of porcine plasma protein and setting on gel properties of surimi produced from fish caught in Thailand. Lwt-Food Sci. Technol. 2004, 37, 177–185. [Google Scholar] [CrossRef]
- Zhou, X.X.; Chen, T.; Lin, H. Physicochemical properties and microstructure of surimi treated with egg white modified by tea polyphenols. Food Hydrocoll. 2019, 90, 82–89. [Google Scholar] [CrossRef]
- Fang, Q.; Shi, L.; Ren, Z. Effects of emulsified lard and TGase on gel properties of threadfin bream (Nemipterus virgatus) surimi. Lwt-Food Sci. Technol. 2021, 146, 9. [Google Scholar] [CrossRef]
- Wu, M.; Wang, J.; Xiong, Y.L. Rheology and microstructure of myofibrillar protein–starch composite gels: Comparison of native and modified starches. Int. J. Biol. Macromol. 2018, 118, 988–996. [Google Scholar] [CrossRef]
- Vargas, B.P.; Einar, L.; Rafael, E. Impacts of fat from ruminants’ meat on cardiovascular health and possible strategies to alter its lipid composition. J. Sci. Food Agric. Abstr. 2017, 7, 1969–1978. [Google Scholar] [CrossRef]
- Kim, T.K.; Lee, M.H.; Yong, H.I. Impacts of fat types and myofibrillar protein on the rheological properties and thermal stability of meat emulsion systems. Food Chem. 2021, 346, 128930. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S.; Smith, A. Effects of pre-emulsifying fat/oil on meat batter stability, texture and microstructure. Int. J. Food Sci. Technol. 2011, 46, 1216–1224. [Google Scholar] [CrossRef]
- Zhou, X.X.; Jiang, S.; Zhao, D.D. Changes in physicochemical properties and protein structure of surimi enhanced with camellia tea oil. Lwt-Food Sci. Technol. 2017, 84, 562–571. [Google Scholar] [CrossRef]
- Zhou, X.X.; Chen, H.; Lyu, F. Physicochemical properties and microstructure of fish myofibrillar protein-lipid composite gels: Effects of fat type and concentration. Food Hydrocoll. 2019, 90, 433–442. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Fat reduction in comminuted meat products-effects of beef fat, regular and pre-emulsified canola oil. Meat Sci. 2011, 87, 356–360. [Google Scholar] [CrossRef]
- Yang, D.; Wu, G.; Li, P. The effect of fatty acid composition on the oil absorption behavior and surface morphology of fried potato sticks via LF-NMR, MRI, and SEM. Food Chem. 2020, 7, 100095. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Marsol-Vall, A.; Heponiemi, P. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Res. Int. 2019, 122, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Lee, M.H.; Yong, H.I. Effect of interaction between mealworm protein and myofibrillar protein on the rheological properties and thermal stability of the prepared emulsion systems. Foods 2020, 10, 1443. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiong, Y.L.; Chen, J. Rheological and microstructural properties of porcine myofibrillar protein–lipid emulsion composite gels. J. Food Sci. 2009, 74, 77–87. [Google Scholar] [CrossRef]
- Ke, L.; Xu, Y.; Gao, G. Catalase to demulsify oil-in-water fish oil-polysorbate emulsion and affect lipid oxidation. Food Res. Int. 2020, 133, 109169. [Google Scholar] [CrossRef]
- Aziz, A.N.; Khan, M.; Ali, F. Effect of protein and oil volume concentrations on emulsifying properties of acorn protein isolate. Food Chem. 2020, 324, 126894. [Google Scholar] [CrossRef]
- Benjakul, S.; Wonnop, V.; Yuwathida, K. The effect of whitening agents on the gel-forming ability and whiteness of surimi. Int. J. Food Sci. Technol. 2004, 39, 773–781. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Jiang, Q. Effect of incorporated surimi on the wheat dough rheological properties and noodle quality. Food Sci. Technol. Res. 2014, 6, 1191–1197. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.; Li, Y.; Wang, C. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 116290. [Google Scholar] [CrossRef]
- Zamani, G.; Ezzatpanah, A.H.; Rajabzadeh, G. Comparison and analysis characteristics of flax, perilla and basil seed oils cultivated in Iran. J. Food Sci. Technol. 2020, 57, 1258–1268. [Google Scholar] [CrossRef]
- Han, Z.Y.; Xu, S.; Sun, J. Effects of fatty acid saturation degree on salt-soluble pork protein conformation and interfacial adsorption characteristics at the oil/water interface. Food Hydrocoll. 2021, 113, 106472. [Google Scholar] [CrossRef]
- Kiokias, S.; Reiffers-Magnani, C.K.; Bot, A. Stability of whey-protein-stabilized oil-in-water emulsions during chilled storage and temperature cycling. J. Agric. Food Chem. 2004, 12, 3823. [Google Scholar] [CrossRef] [PubMed]
- Dybowska, B.E. Model whey protein concentrate-stabilized O/W emulsions with increasing protein concentration. Milchwissenschaft. Milchwissenschaft 2003, 3, 170–173. [Google Scholar]
- Guo, Q.; Mu, T.H. Emulsifying properties of sweet potato protein: Effect of protein concentration and oil volume fraction. Food Hydrocoll. 2011, 25, 98–106. [Google Scholar] [CrossRef]
- Shin, D.J.; Lee, H.J.; Lee, D. Fat replacement in chicken sausages manufactured with broiler and old laying hens by different vegetable oils. Poult. Sci. 2020, 99, 2811–2818. [Google Scholar] [CrossRef]
- Hongsprabhas, P.; Barbut, S. Protein and salt effects on Ca2+ induced cold gelation of whey protein isolate. J. Food Sci. 2010, 62, 382–385. [Google Scholar] [CrossRef]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, X.F.; Chang, T. Effects of vegetable oils on gel properties of surimi gels. Lwt-Food Sci. Technol. 2014, 57, 586–593. [Google Scholar] [CrossRef]
- Gordon, A.; Barbut, S. Raw Meat Batter Stabilization: Morphological study of the role of interfacial protein film. Can. Inst. Food Sci. Technol. J. 1991, 24, 136–142. [Google Scholar] [CrossRef]
- Tadpit, C.K.; Pan, C.P.; Park, J.W. Conformational changes and dynamic rheological properties of fish sarcoplasmic proteins treated at various pH. Food Chem. 2010, 121, 1046–1052. [Google Scholar]
- Alvarez, D.Y.; Xiong, M.C.; Garrido, M.D. Textural and viscoelastic properties of pork frankfurters containing canola-olive oils, rice bran, and walnut. Meat Sci. 2012, 92, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, K.; Wang, Y. Insight into the mechanism of textural deterioration of myofibrillar protein gels at high temperature conditions. Food Chem. 2020, 330, 127186. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, F.X.; Wang, X. Changes of protein secondary structures of pollock surimi gels under high-temperature (100 °C and 120 °C) treatment. Food Eng. 2016, 171, 159–163. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T. Effect of psyllium (Plantago ovata Forks) husk on characteristics, rheological and textural properties of threadfin bream surimi gel. Foods 2021, 6, 1181. [Google Scholar] [CrossRef]





| Palmitic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | Linolenic Acid | |
|---|---|---|---|---|---|
| Perilla seed oil | 8.51% | 1.11% | 19.83% | 15.82% | 54.44% |
| Soybean Oil | 13.66% | 2.03% | 31.77% | 43.82% | 6.44% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Chen, X.; Zheng, J.; Fan, W.; Ding, Y.; Zhou, X. Emulsion Surimi Gel with Tunable Gel Properties and Improved Thermal Stability by Modulating Oil Types and Emulsification Degree. Foods 2022, 11, 179. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11020179
Zhu S, Chen X, Zheng J, Fan W, Ding Y, Zhou X. Emulsion Surimi Gel with Tunable Gel Properties and Improved Thermal Stability by Modulating Oil Types and Emulsification Degree. Foods. 2022; 11(2):179. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11020179
Chicago/Turabian StyleZhu, Shichen, Xiaocao Chen, Jiani Zheng, Wenlong Fan, Yuting Ding, and Xuxia Zhou. 2022. "Emulsion Surimi Gel with Tunable Gel Properties and Improved Thermal Stability by Modulating Oil Types and Emulsification Degree" Foods 11, no. 2: 179. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11020179