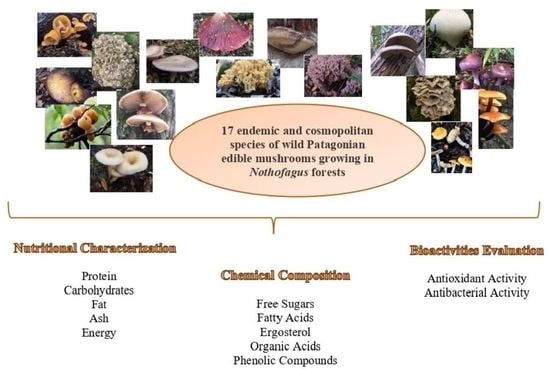
Nutritional Composition and Bioactive Properties of Wild Edible Mushrooms from Native Nothofagus Patagonian Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungi Identification and Sampling
2.2. Nutritional Characterization
2.3. Chemical Composition
2.3.1. Free Sugars
2.3.2. Fatty Acids
2.3.3. Ergosterol
2.3.4. Organic Acids Composition
2.3.5. Phenolic Composition
2.4. Bioactivities Evaluation
2.4.1. Evaluation of Antioxidant Activity
2.4.2. Evaluation of Antibacterial Activity
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boa, E. Wild edible fungi: A global overview of their use and importance to people. In Non-Wood Forest Products; FAO: Rome, Italy, 2004; pp. 1–147. [Google Scholar]
- Guedes de Pinho, P.; Ribeiro, B.; Gonçalves, R.F.; Baptista, P.; Valentão, P.; Seabra, R.M.; Andrade, P.B. Correlation between the pattern volatiles and the overall aroma of wild edible mushrooms. J. Agric. Food Chem. 2008, 56, 1704–1712. [Google Scholar] [CrossRef]
- Barroetaveña, C.; Toledo, C.V. The nutritional benefits of mushrooms. In Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications, Ferreira, I.C.F.R., Morales Gómez, P., Barros, L., Eds.; Wiley-Blackwell: Chichester, UK, 2017; pp. 65–82. [Google Scholar]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Bobek, P.; Galbavy, S. Hypercholesterolemic and anti-atherogenic effect of oyster mushroom (Pleurotus ostreatus) in rabbit. Nahrung 1999, 45, 339–342. [Google Scholar] [CrossRef]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Lindequist, U.; Niedermeyer, T.H.; Jülich, W.D. The pharmacological potential of mushrooms. Evid-Based Complement. Altern Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Zaidman, B.Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef]
- Petrova, R.D.; Reznick, A.Z.; Wasser, S.P.; Denchev, C.M.; Nevo, E.; Mahajna, J. Fungal metabolites modulating NF-kappa B activity: An approach to cancer therapy and chemoprevention (Review). Oncol. Rep. 2008, 19, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, B.; Valentão, P.; Baptista, P.; Seabra, R.M.; Andrade, P.B. Phenolic compounds, organic acids profiles and antioxidative properties of beefsteak fungus (Fistulina hepatica). Food Chem. Toxicol. 2007, 45, 1805–1813. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Venturini, B.A.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Chemical composition and biological properties of Portuguese wild mushrooms: A comprehensive study. J. Agric. Food Chem. 2008, 56, 3856–3862. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional value and biological properties of Chilean wild and commercial edible mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef]
- Barros, L.; Falcao, S.; Baptista, P.; Freire, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008, 111, 61–66. [Google Scholar] [CrossRef]
- MAyDS. Informe Bosque Andino Patagónico. In Segundo Inventario Nacional de Bosques Nativos; Ministerio de Ambiente y Desarrollo Sostenible de la Nación: Buenos Aires, Argentina, 2020; Available online: https://www.argentina.gob.ar/sites/default/files/informe_region_forestal_bosque_andino_patagonico_segunda_revision_0.rar (accessed on 24 May 2022).
- Gamundí, I.J.; Horak, E. Hongos de los Bosques Andino-Patagónicos; Vázquez Mazzini Editores: Buenos Aires, Argentina, 1993; pp. 1–144. [Google Scholar]
- Barroetaveña, C.; Toledo, C. Diversity and ecology of edible mushrooms from Patagonia native forests, Argentina. In Mushrooms, Humans and Nature in a Changing World: Perspectives from Ecological, Agricultural and Social Sciences, 1st ed.; Perez-Moreno, J., Guerin-Laguette, A., Flores Arzú, R., Yu, F., Eds.; Springer: Cham, Switzerland, 2020; pp. 297–318. [Google Scholar]
- Molares, S.; Toledo, C.V.; Stecher, G.; Barroetaveña, C. Traditional mycological knowledge and processes of change in Mapuche communities from Patagonia, Argentina: A study on wild edible fungi in Nothofagaceae forests. Mycologia 2020, 112, 9–23. [Google Scholar] [CrossRef]
- Valenzuela-Flores. Hongos comestibles silvestres colectados en la X región de Chile. Boletín Micológico. 2003, 18, 1–14. [Google Scholar] [CrossRef]
- Toledo, C.V.; Barroetaveña, C.; Rajchenberg, M. Phenology and environmental variables associated to the fruiting of wild edible mushrooms from Andean-Patagonia forests in Argentina. Rev. Mex. Biodiv. 2014, 85, 1093–1103. [Google Scholar] [CrossRef] [Green Version]
- Barroetaveña, C.; Toledo, C.V. Hongos Silvestres Comestibles Novedosos en el Bosque Nativo y en las Plantaciones de Patagonia Andina, Argentina. Cienc. Investig. For. 2016, 22, 73–88. [Google Scholar] [CrossRef]
- González, G.C.; Barroetaveña, C.; Visnovsky, S.B.; Rajchenberg, M.; Pildain, M.B. A new species, phylogeny, and a worldwide key of the edible wood decay Fistulina (Agaricales). Mycol. Prog. 2021, 20, 733–746. [Google Scholar] [CrossRef]
- Toledo, C.; Barroetaveña, C.; Fernandes, A.; Barros, L.; Ferreira, I. Chemical and antioxidant properties of wild edible mushrooms from native Nothofagus spp. forest, Argentina. Molecules 2016, 21, 1201. [Google Scholar] [CrossRef]
- Kostić, M.; Ivanov, M.; Babić, S.S.; Petrović, J.; Soković, M.; Ćirić, A. An up-to-date review on bio-resource therapeutics effective against bacterial species frequently associated with chronic sinusitis and tonsillitis. Curr. Med. Chem. 2020, 27, 6892–6909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, D.; You, Y.; Zeng, S.; Li, Y.; Tang, Q.; Han, G.; Liu, A.; Feng, C.; Li, C.; et al. Nutritional composition of boletus mushrooms from Southwest China and their antihyperglycemic and antioxidant activities. Food Chem. 2016, 211, 83–91. [Google Scholar] [CrossRef]
- Barroetaveña, C.; Pildain, M.B. Review: Edible fungi for local development in the Patagonian Andes of Argentina. For. Syst. 2022, in press.
- Pérez-Moreno, J.; Mortimer, P.E.; Ku, J.; Karunarathna, S.C.; Li, H. Global perspectives on the ecological, cultural and socioeconomic relevance of wild edible fungi. Stud. Fungi 2021, 6, 408–424. [Google Scholar] [CrossRef]
- Rajchenberg, M. Polypores (Basidiomycetes) from the Patagonian Andes Forests of Argentina. In Bibliotheca Mycologica Band 201, J; Cramer Verlag: Stuttgart, Germany, 2006; 300p. [Google Scholar]
- Salgado Salomón, M.E.; Dresch, P.; Horak, E.; Galleguillos, F.; Barroetaveña, C.; Peintner, U. The enigmatic Cortinarius magellanicus complex occurring in Nothofagaceae forests of the Southern Hemisphere. Fungal Biol. 2018, 122, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Salgado Salomón, M.E.; Barroetaveña, C.; Niskanen, T.; Liimatainen, K.; Smith, M.E.; Peintner, U. Loose ends in the Cortinarius phylogeny: Five new myxotelamonoid species indicate a high diversity of these ectomycorrhizal fungi with South American Nothofagaceae. Life 2021, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Rajchenberg, M.; Pildain, M.B.; de Errasti, A.; Riquelme, C.; Becerra, J.; Torres-Díaz, C.; Cabrera-Pardo, J.R. Species and genera in Aleurodiscus sensu lato as viewed from the Southern Hemisphere. Mycologia 2021, 113, 1264–1277. [Google Scholar] [CrossRef]
- Rugolo, M.; Barroetaveña, C.; Barrett, M.D.; Mata, G.; Hood, I.A.; Rajchenberg, M.; Pildain, M.B. Phylogenetic relationships and taxonomy of Grifola (Polyporales). Mycol. Prog. 2022. accepted. [Google Scholar]
- González, G.; Barroetaveña, C.; Visnovsky, S.B.; Rajchenberg, M.; Pildain, M.B. Diversity of the Genus Ramaria in the Patagonian andes Forests of Argentina. Mycol. Prog. 2022. submitted. [Google Scholar]
- AOAC. Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2016. [Google Scholar]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from Portugal by ultra fast liquid chromatography and photodiode array detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem. 2012, 130, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Vieira Junior, W.G.; Centeio Cardoso, R.V.; Fernandes, A.; Ferreira, I.C.F.R.; Barros, L.; Pardo-Giménez, A.; Soares, D.M.M.; Stevani, C.V.; Zied, D.C. Influence of strains and environmental cultivation conditions on the bioconversion of ergosterol and vitamin D2 in the sun mushroom. J. Sci. Food Agric. 2021, 102, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.V.C.; Carocho, M.; Fernandes, A.; Cunha Zied, D.; Cobos, J.D.V.; González-Paramás, A.M.; Ferreira, I.C.F.R.; Barros, L. Influence of calcium silicate on the chemical properties of Pleurotus ostreatus var. florida (Jacq.) P. Kumm. J. Fungi 2020, 6, 299. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb. f.: An underexploited and highly disseminated species. Ind. Crop. Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Mandim, F.; Barros, L.; Calhelha, R.C.; Abreu, R.M.V.; Pinela, J.; Alves, M.J.; Heleno, S.; Santos, P.F.; Ferreira, I.C.F.R. Calluna vulgaris (L.) Hull: Chemical characterization, evaluation of its bioactive properties and effect on the vaginal microbiota. Food Funct. 2019, 10, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crop. Prod. 2018, 128, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. Cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Heleno, S.A.; Ferreira, I.C.F.R.; Esteves, A.P.; Ćirić, A.; Glamočlija, J.; Martins, A.; Soković, M.; Queiroz, M.J.R.P. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef]
- R Studio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 2 July 2022).
- Schmeda-Hirschmann, G.; Razmilic, I.; Gutierrez, M.I.; Loyola, J.I. Proximate composition and biological activity of food plants gathered by chilean Amerindians. Econ. Bot. 1999, 53, 177–187. [Google Scholar] [CrossRef]
- Chang, S.T.; Miles, P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 27–52. [Google Scholar] [CrossRef]
- Richards, A.B.; Krakowka, S.; Dexter, L.B.; Schmid, H.; Wolterbeek, A.P.M.; Waalkens-Berendsen, D.H.; Shigoyuki, A.; Kurimoto, M. Trehalose: A review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 2002, 40, 871–898. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Kölker, S. Organic acid disorders. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: London, UK, 2014; pp. 688–693. [Google Scholar]
- Puiggrós, C.; Chacón, P.; Armadans, L.I.; Clapés, J.; Planas, M. Effects of oleic-rich and omega-3-rich diets on serum lipid pattern and lipid oxidation in mildly hypercholesterolemic patients. Clin. Nutr. 2002, 21, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.M.; López, S.; Bermúdez, B.; Abia, R.; Villar, J.; Muriana, F.J.G. A meal rich in oleic acid beneficially modulates postprandial sICAM-1 and sVCAM-1 in normotensive and hypertensive hypertriglyceridemic subjects. J. Nutr. Biochem. 2008, 19, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Sande, D.; Oliveira, G.P.; Moura, M.A.F.E.; Martins, B.A.; Lima, M.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Aires, D.; Capdevila, N.; Segundo, M.J. Ácidos grasos esenciales su influencia en las diferentes etapas de la vida. Offarm Farm. Y Soc. 2005, 24, 96–102. [Google Scholar]
- Lv, W.; Xu, D. Docosahexaenoic acid delivery systems, bioavailability, functionality and applications: A review. Foods 2022, 11, 2685. [Google Scholar] [CrossRef]
- Martínez-Crovetto, R. Estudios Etnobotánicos 4: Nombres de plantas y su utilidad, según los indios Onas de Tierra del Fuego. Bonplandia 1968, 3, 1–20. [Google Scholar] [CrossRef]
- Domínguez Díaz, E. Flora de interés etnobotánico usada por los pueblos originarios: Aónikenk, Selk’nam, Kawésqar, Yagan y Haush en la Patagonia Austral. Dominguezia 2010, 26, 19–29. [Google Scholar]
- Jasinghe, V.J.; Perera, C.O. Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chem. 2005, 92, 541–546. [Google Scholar] [CrossRef]
- Espinosa, N.A.Z.; Velásquez, J.M.A.; González, V.B.; Blanco, K.E.J.; Maya, G.C. Vitamin D: New paradigms. Med. Lab. 2011, 17, 211–246. [Google Scholar]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC-DAD-ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Correia, D.; Casal, S.; Oliveira, B.; Ferreira, I.C.F.R. Fatty acid and sugar compositions and nutritional value of five edible mushrooms from Northeast Portugal. Food Chem. 2007, 105, 140–145. [Google Scholar] [CrossRef]
- Bruijn, J.; Loyola, C.; Aqueveque, P.; Cañumir, J.; Cortéz, M.; France, A. Influence of heat treatment on the antioxidant properties of Grifola gargal hydro-alcoholic extracts. Micol. Apl. Int. 2008, 20, 27–34. [Google Scholar]
- Bruijn, J.; Loyola, C.; Aqueveque, P.; Cañumir, J.; Cortéz, M.; France, A. Antioxidant properties of extracts obtained from Grifola gargal mushrooms. Micol. Apl. Int. 2009, 21, 11–18. [Google Scholar]
- Postemsky, P.D.; Curvetto, N.R. Enhancement of wheat grain antioxidant activity by Solid-state fermentation with Grifola spp. J. Med. Food. 2014, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]

| Species | Total Fat | Crude Protein | Carbohydrates | Ash | Energy |
|---|---|---|---|---|---|
| A. vitellinus | 4.70 ± 0.20b | 5.26 ± 0.01kl | 81.00 ± 1.00c | 8.90 ± 0.30ef | 387.74 ± 0.04bc |
| C. magellanicus | 4.40 ± 0.20b | 14.40 ± 0.10e | 49.00 ± 2.00i | 32.00 ± 1.00a | 293.00 ± 4.00i |
| C. xiphidipus | 2.01 ± 0.04e | 12.30 ± 0.40fg | 74.80 ± 0.10d | 10.9 ± 0.30d | 366.00 ± 1.00f |
| C. aegerita | 2.60 ± 0.20d | 10.70 ± 0.30h | 63.00 ± 1.00f | 23 ± 1.00b | 320.00 ± 5.00h |
| C. hariotii | 2.60 ± 0.20d | 5.87 ± 0.07jk | 86.20 ± 0.20b | 5.3 ± 0.30h | 392.00 ± 2.00ab |
| F. antarctica | 0.70 ± 0.02f | 3.20 ± 0.10m | 89.70 ± 0.20a | 6.4 ± 0.30g | 378.00 ± 1.00de |
| F. endoxantha | 0.77 ± 0.04f | 4.70 ± 0.20l | 80.00 ± 1.00 c | 15 ± 1.00c | 345.00 ± 2.00g |
| F. pumiliae | 1.02 ± 0.02f | 6.53 ± 0.04j | 82.00 ± 1.00c | 10 ± 1.00de | 364.00 ± 2.00f |
| F. velutipes | 1.70 ± 0.01e | 11.40 ± 0.30gh | 66.00 ± 1.00f | 21.20 ± 0.40b | 324.00 ± 2.00h |
| G. gargal | 5.90 ± 0.30a | 5.20 ± 0.10kl | 81.10 ± 0.10c | 7.90 ± 0.40f | 398.00 ± 3.00a |
| G. sordulenta | 1.90 ± 0.10e | 12.60 ± 0.10f | 71.00 ± 1.00e | 14.00 ± 1.00c | 353.00 ± 3.00g |
| H. dusenii | 3.10 ± 0.10cd | 22.00 ± 1.00c | 63.00 ± 1.00f | 11.30 ± 0.30d | 370.40 ± 0.40ef |
| L. nuda | 3.30 ± 0.20c | 30.30 ± 0.10b | 57.60 ± 0.20g | 8.70 ± 0.40f | 382.00 ± 3.00cd |
| L. perlatum | 3.32 ± 0.10c | 36.60 ± 0.10a | 52.00 ± 0.30h | 8.10 ± 0.40f | 384.00 ± 2.00bcd |
| P. ostreatus | 1.05 ± 0.05f | 7.65 ± 0.01i | 86.53 ± 0.01b | 4.80 ± 0.10h | 386.00 ± 1.00bcd |
| R. botrytis | 3.40 ± 0.10c | 12.60 ± 0.40f | 73.00 ± 1.00de | 11.00 ± 1.00d | 373.00 ± 1.00ef |
| R. patagonica | 0.90 ± 0.02f | 18.10 ± 0.20d | 72.40 ± 0.30de | 8.60 ± 0.10f | 370.00 ± 1.00ef |
| Organic Acids | Sugars | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Oxalic | Quinic | Malic | Shikimic | Citric | Succinic | Fumaric | Not Identified | Fructose | Mannitol | Trehalose |
| A. vitellinus | nd | nd | 74.58 ± 1.03a | nd | 31.10 ± 0.40b | 452.00 ± 2.00a | 0.05 ± 0.01n | 0.17 ± 0.01i | nd | 8.80 ± 0.10a | 3.16 ± 0.03hi |
| C. magellanicus | 2.02 ± 0.05fg | nd | 10.90 ± 0.30f | nd | 57.40 ± 0.03a | 8.10 ± 0.20d | 11.02 ± 0.02a | 0.15 ± 0.01i | nd | 4.80 ± 0.20c | 3.04 ± 0.12hi |
| C. xiphidipus | 0.93 ± 0.01gh | 542.00 ± 3.00a | nd | nd | nd | nd | 3.93 ± 0.01c | nd | 0.70 ± 0.01e | 3.30 ± 0.10d | 17.60 ± 0.40a |
| C. aegerita | 0.49 ± 0.01jk | nd | 17.29 ± 0.04d | 0.72 ± 0.04b | nd | nd | 3.40 ± 0.02e | nd | 0.31 ± 0.02f | 1.22 ± 0.04g | 13.54 ± 0.02c |
| C. hariotii | 0.09 ± 0.01m | nd | 13.40 ± 0.30e | nd | 23.40 ± 0.20a | nd | 2.39 ± 0.01i | 3.19 ± 0.02d | 2.10 ± 0.10c | 0.33 ± 0.01j | 2.10 ± 0.10j |
| F. antarctica | 0.05 ± 0.02m | 0.50 ± 0.10b | 13.60 ± 0.30e | nd | nd | nd | 2.54 ± 0.01h | 12.00 ± 1.00a | 10 ± 1.00b | 1.00 ± 0.10h | 8.00 ± 1.00e |
| F. endoxantha | 0.35 ± 0.01lm | nd | 16.80 ± 1.10d | nd | nd | nd | 5.34 ± 0.04b | 7.00 ± 0.40b | 23.18 ± 1.02a | nd | 3.70 ± 0.10h |
| F. pumiliae | 0.44 ± 0.01kl | nd | 35.00 ± 1.00b | nd | nd | nd | 5.33 ± 0.01b | 6.60 ± 0.30b | 9.10 ± 0.40b | 3.40 ± 0.10d | 5.30 ± 0.20fg |
| F. velutipes | 3.90 ± 0.10cd | nd | 32.60 ± 0.50b | nd | nd | nd | 5.40 ± 0.02b | 1.87 ± 0.01e | 8.60 ± 0.10b | 1.64 ± 0.02f | 4.70 ± 0.40g |
| G. gargal | 2.40 ± 0.10ef | nd | 1.90 ± 0.02h | nd | nd | nd | 0.34 ± 0.01m | 0.16 ± 0.01i | 0.77 ± 0.04d | 0.25 ± 0.01k | 2.50 ± 0.10ij |
| G. sordulenta | 0.72 ± 0.02hi | nd | 21.40 ± 0.30c | nd | nd | nd | 2.79 ± 0.02g | nd | 0.30 ± 0.01f | 0.50 ± 0.02i | 11.30 ± 0.20d |
| H. dusenii | 3.39 ± 0.03de | nd | nd | nd | nd | nd | 1.74 ± 0.01j | 0.26 ± 0.02h | 0.18 ± 0.01g | 2.30 ± 0.10e | 5.70 ± 0.10f |
| L. nuda | 66.70 ± 1.90a | 488.00 ± 38.00a | nd | nd | nd | 277.00 ± 2.00b | 1.31 ± 0.01k | 0.57 ± 0.01g | 0.17 ± 0.01g | 1.29 ± 0.01g | 7.10 ± 0.10e |
| L. perlatum | 4.90 ± 0.10def | nd | 8.70 ± 0.20g | nd | nd | 87.00 ± 3.00c | 1.20 ± 0.10l | 3.88 ± 0.03c | nd | nd | 5.50 ± 0.10fg |
| P. ostreatus | 0.59 ± 0.03ij | nd | 11.90 ± 0.30ef | 1.07 ± 0.03a | nd | 83.00 ± 1.00c | 2.54 ± 0.01h | nd | nd | 3.30 ± 0.10d | 15.80 ± 0.40b |
| R. botrytis | 25.36 ± 0.02b | nd | nd | nd | nd | nd | 3.02 ± 0.02f | 1.59 ± 0.05f | 0.76 ± 0.04de | 6.34 ± 0.04b | Nd |
| R. patagonica | 25.60 ± 0.10ab | nd | nd | nd | 57.30 ± 0.80c | nd | 3.67 ± 0.01d | nd | 0.86 ± 0.04d | 8.60 ± 0.40a | Nd |
| Species | A. vitellinus | C. magellanicus | C. xiphidipus | C. aegerita | C. hariotii | F. antarctica | F. endoxantha | F. pumiliae | F. velutipes |
|---|---|---|---|---|---|---|---|---|---|
| C8:0 | nd | 0.63 ± 0.02b | nd | 0.05 ± 0.01d | nd | nd | nd | 0.81 ± 0.02a | nd |
| C10:0 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| C11:0 | nd | nd | nd | nd | nd | nd | 1.50 ± 0.03a | 0.86 ± 0.04b | 1.70 ± 0.03a |
| C12:0 | nd | 0.11 ± 0.01c | nd | 0.10 ± 0.01c | nd | 0.28 ± 0.01b | nd | nd | nd |
| C13:0 | 0.15 ± 0.04cd | 0.37 ± 0.01a | 0.18 ± 0.01bcd | 0.24 ± 0.01b | 0.13 ± 0.01d | nd | nd | nd | nd |
| C14:0 | 0.28 ± 0.01g | 0.35 ± 0.01f | 0.23 ± 0.01gh | 0.37 ± 0.01ef | 0.22 ± 0.01hi | 1.33 ± 0.02b | 1.80 ± 0.05ab | 2.4 ± 0.10a | nd |
| C15:0 | 0.82 ± 0.01g | 1.47 ± 0.03d | 0.50 ± 0.01i | 0.44 ± 0.01j | 0.15 ± 0.01l | 0.71 ± 0.01g | 1.58 ± 0.04c | 1.66 ± 0.04c | nd |
| C16:0 | 21.90 ± 0.1d | 53.40 ± 0.20b | 17.00 ± 1.00f | 19.60 ± 0.10e | 17.30 ± 0.20f | 18.30 ± 0.10e | 51.10 ± 0.70b | 52.6 ± 0.50b | 61.00 ± 1.00a |
| C16:1 | 1.70 ± 0.04a | 0.61 ± 0.02d | 0.31 ± 0.01f | 0.57 ± 0.01d | nd | 0.63 ± 0.03d | 1.40 ± 0.01b | nd | nd |
| C17:0 | 0.16 ± 0.01h | nd | 0.14 ± 0.01i | 0.21 ± 0.01g | 0.19 ± 0.01g | nd | 3.19 ± 0.05c | 5.4 ± 0.20a | nd |
| C18:0 | 4.40 ± 0.01de | 7.49 ± 0.02b | 3.2 ± 0.10h | 5.10 ± 0.10c | 4.65 ± 0.03cd | 2.92 ± 0.02h | 7.10 ± 0.30b | nd | nd |
| C18:1n9t | nd | nd | nd | nd | nd | nd | nd | 5.9 ± 0.10c | nd |
| C18:1n9c | 52.60 ± 0.10b | 19.9 ± 0.10l | 31.30 ± 0.50g | 20.2 ± 0.20k | 6.50 ± 0.10m | 26.80 ± 0.30j | 32.40 ± 0.30f | 30.3 ± 0.10h | nd |
| C18:2n6t | 0.22 ± 0.01f | nd | 1.3 ± 0.10c | 1.75 ± 0.02b | 0.58 ± 0.01d | nd | nd | nd | 37.00 ± 10.00 a |
| C18:2n6c | 13.50 ± 0.10j | 12.3 ± 0.10k | 40.4 ± 0.30e | 44.70 ± 0.20d | 25.10 ± 0.20i | 48.30 ± 0.20c | nd | nd | nd |
| C18:3n3 | 0.96 ± 0.01b | nd | nd | 0.14 ± 0.01d | 12.50 ± 0.10a | nd | nd | nd | nd |
| C20:0 | 0.74 ± 0.02f | 1.10 ± 0.01bc | 0.80 ± 0.01de | 0.77 ± 0.01ef | 2.90 ± 0.10a | nd | nd | nd | nd |
| C20:1 | 0.22 ±0.01h | nd | 0.75 ± 0.02d | 0.67 ± 0.01e | 0.43 ± 0.01f | nd | nd | nd | nd |
| C20:2 | 0.73 ± 0.04d | nd | 0.85 ± 0.02d | 1.10 ± 0.01c | 1.50 ± 0.10b | nd | nd | nd | nd |
| C20:4n6 | nd | nd | nd | nd | 0.44 ± 0.01 | nd | nd | nd | nd |
| C20:5n3 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| C21:0 | nd | nd | nd | 0.69 ± 0.01a | 0.25 ± 0.01b | nd | nd | nd | nd |
| C22:0 | 0.55 ± 0.01h | nd | 1.11 ± 0.01c | 0.79 ± 0.01f | 7.40 ± 0.10a | 0.46 ± 0.01h | nd | nd | nd |
| C22:1 | nd | 1.8 ± 0.10a | 0.24 ± 0.01b | 0.29 ± 0.01b | 0.27 ± 0.01b | nd | nd | nd | nd |
| C22:2 | nd | nd | nd | 0.33 ± 0.01c | 0.46 ± 0.01b | nd | nd | nd | nd |
| C23:0 | 0.19 ± 0.01d | 0.42 ± 0.01c | nd | nd | nd | nd | nd | nd | |
| C24:0 | 0.47 ± 0.01f | nd | 0.53 ± 0.01f | 0.90 ± 0.02d | 6.75 ± 0.02a | 0.44 ± 0.01f | nd | nd | nd |
| C24:1 | 0.07 ± 0.01g | nd | 0.71 ± 0.02c | 0.36 ± 0.01d | 4.47 ± 0.05a | nd | nd | nd | nd |
| C22:6n3 | 0.27 ± 0.01d | nd | 0.38 ± 0.01c | 0.69 ± 0.02b | 7.74 ± 0.01a | nd | nd | nd | nd |
| SFAs | 29.70 ± 0.10gh | 65.40 ± 0.20b | 23.70 ± 0.40i | 29.20 ± 0.01h | 39.90 ± 0.20ef | 24.40 ± 0.05i | 66.20 ± 0.30a | 63.80 ± 0.20c | 63.00 ± 1.00cd |
| MUFAs | 54.60 ± 0.10ab | 22.40 ± 0.10i | 33.00 ± 1.00g | 22.10 ± 0.20i | 11.70 ± 0.10j | 27.30 ± 0.20h | 33.80 ± 0.30g | 36.20 ± 0.20ef | nd |
| PUFAs | 15.73 ± 0.01i | 12.30 ± 0.10j | 43.00 ± 0.20d | 48.70 ± 0.20c | 48.40 ± 0.20c | 48.30 ± 0.20c | nd | nd | 37.00 ± 1.00f |
| Species | G. gargal | G. sordulenta | H. dusenii | L. nuda | L. perlatum | P. ostreatus | R. botrytis | R. patagonica | |
| C8:0 | nd | nd | 0.25 ± 0.01c | Nd | nd | nd | nd | nd | |
| C10:0 | nd | nd | Nd | nd | nd | 0.40 ± 0.02 | nd | nd | |
| C11:0 | nd | nd | Nd | nd | nd | 0.84 ± 0.01b | nd | 0.17 ± 0.01c | |
| C12:0 | nd | 0.23 ± 0.01b | 0.11 ± 0.01c | nd | nd | 0.37 ± 0.01a | nd | nd | |
| C13:0 | nd | 0.20 ± 0.01bc | 0.15 ± 0.01cd | nd | nd | 0.41 ± 0.02a | nd | nd | |
| C14:0 | nd | 1.20 ± 0.04bc | 0.36 ± 0.01f | 0.36 ± 0.01f | 0.47 ± 0.01de | 0.77 ± 0.02cd | nd | 0.17 ± 0.01i | |
| C15:0 | nd | 3.23 ± 0.03b | 1.12 ± 0.01e | 0.30 ± 0.01k | nd | 5.40 ± 0.10a | 0.86 ± 0.01f | 0.60 ± 0.01h | |
| C16:0 | 20.00 ± 1.00e | 21.33 ± 0.03d | 27.30 ± 0.1c | 15.40 ± 0.20g | 17.00 ± 1.00f | 53.00 ± 1.00b | 9.67 ± 0.02i | 13.90 ± 0.10h | |
| C16:1 | 0.63 ± 0.01d | nd | Nd | 0.74 ± 0.02c | nd | nd | nd | 0.43 ± 0.01e | |
| C17:0 | nd | 1.46 ± 0.04d | 0.49 ± 0.02f | nd | nd | 4.20 ± 0.20b | 1.50 ± 0.02d | 0.77 ± 0.01e | |
| C17:1 | nd | nd | Nd | nd | nd | nd | 0.30 ± 0.01a | 0.29 ± 0.01a | |
| C18:0 | 8.90 ± 0.10a | 3.94 ± 0.05fg | 9.32 ± 0.03a | 2.05 ± 0.05i | 3.19 ± 0.05h | nd | 4.01 ± 0.01ef | 3.70 ± 0.10g | |
| C18:1n9t | nd | nd | Nd | nd | 11.50 ± 0.10a | 7.43 ± 0.02b | nd | nd | |
| C18:1n9c | 58.20 ± 0.20a | 28.90 ± 0.10i | 46.80 ± 0.03c | 25.50 ± 0.10j | nd | 27.00 ± 1.00j | 42.67 ± 0.01d | 37.60 ± 0.10e | |
| C18:2n6t | nd | 0.39 ± 0.02e | Nd | nd | nd | nd | 0.19 ± 0.01g | nd | |
| C18:2n6c | 11.10 ± 0.30l | 31.45 ± 0.01h | 1.53 ± 0.04m | 54.30 ± 0.10b | 68.00 ± 1.00a | nd | 35.04 ± 0.01g | 38.14 ± 0.02f | |
| C18:3n3 | 0.50 ± 0.02c | nd | Nd | nd | nd | nd | nd | nd | |
| C20:0 | nd | 2.92 ± 0.01a | 1.78 ± 0.01b | nd | nd | nd | 0.89 ± 0.01cd | nd | |
| C20:1 | nd | 1.50 ± 0.03b | 3.48 ± 0.04a | nd | nd | nd | 0.33 ± 0.01g | 0.88 ± 0.04c | |
| C20:2 | nd | nd | 1.76 ± 0.01a | nd | nd | nd | nd | nd | |
| C20:4n6 | nd | nd | Nd | nd | nd | nd | nd | nd | |
| C20:5n3 | nd | nd | Nd | nd | nd | nd | 0.29 ± 0.01 | nd | |
| C21:0 | nd | nd | Nd | nd | nd | nd | nd | nd | |
| C22:0 | 0.33 ± 0.01i | 0.97 ± 0.01d | 0.73 ± 0.01g | 0.55 ± 0.01h | nd | nd | 1.56 ± 0.01b | 0.95 ± 0.02e | |
| C22:1 | nd | nd | Nd | nd | nd | nd | 0.48 ± 0.01ab | nd | |
| C22:2 | nd | 1.15 ± 0.04a | Nd | nd | nd | nd | 0.25 ± 0.01d | nd | |
| C23:0 | nd | nd | 0.28 ± 0.01d | nd | nd | nd | 0.78 ± 0.01b | 1.15 ± 0.01a | |
| C24:0 | 0.51 ± 0.02f | 0.99 ± 0.01cd | 1.62 ± 0.01b | 0.50 ± 0.02f | nd | nd | 0.71 ± 0.02e | 1.03 ± 0.02c | |
| C24:1 | nd | nd | 2.32 ± 0.01b | 0.33 ± 0.01d | nd | nd | 0.14 ± 0.01f | 0.18 ± 0.01e | |
| C22:6n3 | 0.12 ± 0.01f | 0.14 ± 0.01e | 0.65 ± 0.01b | nd | nd | nd | 0.34 ± 0.01c | 0.08 ± 0.02g | |
| SFA | 29.40 ± 0.40h | 36.47 ± 0.03fg | 43.47 ± 0.04de | 19.10 ± 0.20k | 20.66 ± 1.00 j | 66.00 ± 1.00b | 19.98 ± 0.01j | 22.42 ± 0.02i | |
| MUFA | 58.90 ± 0.20a | 30.40 ± 0.10h | 52.60 ± 0.01bc | 26.60 ± 0.10h | 11.50 ± 0.10j | 34.00 ± 1.00f | 43.91 ± 0.01cd | 39.37 ± 0.01de | |
| PUFA | 11.70 ± 0.30k | 33.10 ± 0.10h | 3.93 ± 0.04l | 54.30 ± 0.10b | 68.00 ± 1.00a | nd | 36.11 ± 0.02g | 38.22 ± 0.02e | |
| Species | Ergosterol |
|---|---|
| A. vitellinus | 21.50 ± 0.40defg |
| C. magellanicus | 38.00 ± 9.00de |
| C. xiphidipus | 34.00 ± 9.00def |
| C. aegerita | 72.00 ± 10.00b |
| C. hariotii | 0.40 ± 0.10g |
| F. antarctica | 16.00 ± 1.00efg |
| F. endoxantha | 45.00 ± 1.00cd |
| F. pumiliae | 71.00 ± 14.00b |
| F. velutipes | 32.00 ± 2.00defg |
| G. gargal | 123.57 ± 12.00a |
| G. sordulenta | 29.00 ± 9.00def |
| H. dusenii | 16.00 ± 1.00efg |
| L. nuda | 13.00 ± 0.40efg |
| L. perlatum | 31.00 ± 1.00def |
| P. ostreatus | 32.00 ± 2.00defg |
| R. botrytis | 68.50 ± 0.30bc |
| R. patagonica | 13.00 ± 1.00fg |
| Species | p-Coumaric Acid | Gallic Acid | p-Hidroxibenzoic Acid | Protocatechuic Acid | 3-(3,4-Dihydroxyphenyl)-lactic Acid | Galic Acid Monohidrate | Total Phenols |
|---|---|---|---|---|---|---|---|
| A. vitellinus | nd | 1.94 + 0.05h | Nd | nd | nd | nd | 1.94 + 0.05i |
| C. magellanicus | nd | 5.79 + 0.23c | 1.34 + 0.02e | 0.85 + 0.01e | nd | nd | 7.90 + 0.20d |
| C. xiphidipus | nd | 7.36 + 0.07a | 1.60 + 0.10d | 1.16 + 0.02d | nd | nd | 10.20 + 0.10c |
| C. aegerita | 0.83 + 0.01b | 4.91 + 0.04d | 4.10 + 0.10c | 0.69 + 0.01f | nd | 1.19 + 0.04 | 11.70 + 0.10b |
| C. hariotii | nd | 0.80 + 0.01j | Nd | nd | nd | nd | 0.80 + 0.01l |
| F. antarctica | nd | 1.00 + 0.05j | Nd | 0.15 + 0.01h | 0.10 + 0.01c | nd | 1.30 + 0.10k |
| F. endoxantha | nd | 2.03 + 0.04h | 5.90 + 0.10b | 7.65 + 0.04a | 1.75 + 0.02a | nd | 11.40 + 0.10b |
| F. pumiliae | nd | 1.43 + 0.01i | 0.55 + 0.02g | 0.69 + 0.01f | 1.01 + 0.02b | nd | 3.12 + 0.02h |
| F. velutipes | nd | 2.84 + 0.04g | Nd | nd | nd | nd | 2.84 + 0.04h |
| G. gargal | nd | 3.58 + 0.03f | Nd | 0.56 + 0.02g | nd | nd | 4.20 + 0.10g |
| G. sordulenta | nd | 1.74 + 0.11hi | nd | nd | nd | nd | 1.70 + 0.10ij |
| H. dusenii | nd | 4.40 + 0.15e | nd | nd | nd | nd | 4.40 + 0.12fg |
| L. nuda | 0.22 + 0.01c | 3.61 + 0.05f | 0.87 + 0.02f | nd | nd | nd | 4.70 + 0.10f |
| L. perlatum | 4.32 + 0.04a | 6.75 + 0.13b | 40.30 + 0.30a | nd | nd | nd | 51.40 + 0.20a |
| P. ostreatus | nd | 0.80 + 0.03j | 0.56 + 0.02g | nd | nd | 1.40 + 0.10jk | |
| R. botrytis | nd | 3.84 + 0.12f | nd | 2.90 + 0.10c | nd | nd | 6.80 + 0.20e |
| R. patagonica | nd | 2.54 + 0.04g | nd | 5.30 + 0.07b | nd | nd | 7.80 + 0.10d |
| Species | TBARS | OxHLIA |
|---|---|---|
| A. vitellinus | 551.00 ± 9.00hi | 113.00 ± 7.00i |
| C. magellanicus | 688.00 ± 268.00hi | Na |
| C. xiphidipus | 1206.00 ± 200.00def | 672.00 ± 9.00b |
| C. aegerita | 2426.00 ± 50.00ab | 202.00 ± 14.00g |
| C. hariotii | 1468.00 ± 82.00cde | Na |
| F. antarctica | 2627.00 ± 189.00a | 1066.00 ± 81.00a |
| F. endoxantha | 1132.00 ± 8.00ef | 335.00 ± 8.00de |
| F. pumiliae | 1019.00 ± 20.00fg | 285.00 ± 13.00ef |
| F. velutipes | 1543.00 ± 100.00bcd | 426.00 ± 53.00c |
| G. gargal | 633.00 ± 15.00h | 376.00 ± 17.00cd |
| G. sordulenta | 299.00 ± 31.00ij | 155.00 ± 7.00h |
| H. dusenii | 610.00 ± 17.00h | 220.00 ± 11.00g |
| L. nuda | 711.00 ± 185.00gh | 93.00 ± 6.00i |
| L. perlatum | 1217.00 ± 564.00def | 90.00 ± 4.00i |
| P. ostreatus | 2052.00 ± 276.00abc | Na |
| R. botrytis | 167.00 ± 14.00j | 249.00 ± 12.00fg |
| R. patagonica | 156.00 ± 12.00j | Na |
| Control/Trolox | 18.40 ± 0.10k | 21.80 ± 0.30j |
| Positive Control | |||||||||||||||||||
| AV | CM | CX | CA | CH | Streptomicin 1 mg/mL | Methicilin 1 mg/mL | Ampicillin 10 mg/mL | ||||||||||||
| Antibacterial Activity | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |||
| Gram-negative bacteria | |||||||||||||||||||
| Enterobacter cloacae | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |||
| Escherichia coli | 5 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | >10 | >10 | 0.01 | 0.01 | n.t. | n.t. | 0.15 | 0.15 | |||
| Pseudomonas aeruginosa | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0.06 | 0.06 | n.t. | n.t. | 0.63 | 0.63 | |||
| Salmonella enterocolitica | 2.5 | >10 | 5 | >10 | 5 | >10 | 5 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |||
| Yersinia enterocolitica | 1.25 | >10 | >10 | >10 | 10 | >10 | >10 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |||
| Gram-positive bacteria | |||||||||||||||||||
| Bacillus cereus | 10 | >10 | 10 | >10 | 10 | >10 | >10 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t. | n.t. | n.t. | |||
| Listeria monocytogenes | 10 | >10 | 10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |||
| Staphylococcus aureus | 1.25 | >10 | 5 | >10 | 0.6 | >10 | 1.25 | >10 | 10 | >10 | 0.007 | 0.007 | 0.007 | 0.007 | 0.15 | 0.15 | |||
| Ketaconazole 1mg/mL | |||||||||||||||||||
| Antifungal Activity | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |||||||
| Aspergillus brasiliensis | 10 | >10 | 2.5 | >10 | 2.5 | >10 | 2.5 | >10 | 5 | >10 | 0.06 | 0.125 | |||||||
| Aspergillus fumigatus | 0.07 | 0.15 | 0.07 | 0.15 | 0.07 | 0.15 | >10 | >10 | >10 | >10 | 0.5 | 1 | |||||||
| Positive Control | |||||||||||||||||||
| FA | FE | FP | FV | GG | GS | Streptomicin 1 mg/mL | Methicilin 1 mg/mL | Ampicillin 10 mg/mL | |||||||||||
| Antibacterial Activity | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Gram-negative bacteria | |||||||||||||||||||
| Enterobacter cloacae | 10 | >10 | >10 | >10 | 10 | >10 | >10 | >10 | 10 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |
| Escherichia coli | 10 | >10 | >10 | >10 | 10 | >10 | >10 | >10 | >10 | >10 | 10 | >10 | 0.01 | 0.01 | n.t. | n.t. | 0.15 | 0.15 | |
| Pseudomonas aeruginosa | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0.06 | 0.06 | n.t. | n.t. | 0.63 | 0.63 | |
| Salmonella enterocolitica | 5 | >10 | 10 | >10 | 5 | >10 | 5 | >10 | 10 | >10 | 5 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |
| Yersinia enterocolitica | 10 | >10 | 10 | >10 | 5 | >10 | 2.5 | >10 | 2.5 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |
| Gram-positive bacteria | |||||||||||||||||||
| Bacillus cereus | >10 | >10 | >10 | >10 | 5 | >10 | 5 | >10 | 10 | >10 | >10 | >10 | 0.007 | 0.007 | n.t. | n.t. | n.t. | n.t. | |
| Listeria monocytogenes | 10 | >10 | 5 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | >10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |
| Staphylococcus aureus | 1.25 | >10 | 0.6 | >10 | 0.6 | >10 | 0.6 | >10 | 10 | >10 | 0.3 | >10 | 0.007 | 0.007 | 0.007 | 0.007 | 0.15 | 0.15 | |
| Ketaconazole 1 mg/mL | |||||||||||||||||||
| Antifungal Activity | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |||||
| Aspergillus brasiliensis | 2.5 | >10 | 5 | >10 | 2.5 | >10 | 1.25 | >10 | 5 | >10 | 5 | >10 | 0.06 | 0.125 | |||||
| Aspergillus fumigatus | >10 | >10 | 0.07 | 0.15 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0.5 | 1 | |||||
| Positive Control | |||||||||||||||||||
| HD | LN | LP | PO | RB | RP | Streptomicin 1 mg/mL | Methicilin 1 mg/mL | Ampicillin 10 mg/mL | |||||||||||
| Antibacterial Activity | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Gram-negative bacteria | |||||||||||||||||||
| Enterobacter cloacae | 10 | >10 | >10 | >10 | 10 | >10 | >10 | >10 | 10 | >10 | >10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |
| Escherichia coli | 10 | >10 | 10 | >10 | >10 | >10 | 5 | >10 | 10 | >10 | 10 | >10 | 0.01 | 0.01 | n.t. | n.t. | 0.15 | 0.15 | |
| Pseudomonas aeruginosa | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0.06 | 0.06 | n.t. | n.t. | 0.63 | 0.63 | |
| Salmonella enterocolitica | 5 | >10 | 5 | >10 | 10 | >10 | 5 | >10 | 5 | >10 | 5 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |
| Yersinia enterocolitica | 5 | >10 | 5 | >10 | >10 | >10 | 2.5 | >10 | 1.25 | >10 | 5 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |
| Gram-positive bacteria | |||||||||||||||||||
| Bacillus cereus | 10 | >10 | 5 | >10 | 5 | >10 | >10 | >10 | 10 | >10 | 5 | >10 | 0.007 | 0.007 | n.t. | n.t. | n.t. | n.t. | |
| Listeria monocytogenes | >10 | >10 | 10 | >10 | 5 | >10 | 10 | >10 | >10 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 | |
| Staphylococcus aureus | 0.6 | >10 | 0.6 | >10 | 2.5 | >10 | 0.6 | >10 | 0.3 | >10 | 2.5 | >10 | 0.007 | 0.007 | 0.007 | 0.007 | 0.15 | 0.15 | |
| Ketaconazole 1 mg/mL | |||||||||||||||||||
| Antifungal Activity | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |||||
| Aspergillus brasiliensis | 1.25 | >10 | 2.5 | >10 | 2.5 | >10 | 2.5 | >10 | 5 | >10 | 5 | >10 | 0.06 | 0.125 | |||||
| Aspergillus fumigatus | 0.07 | 0.15 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0.07 | 0.15 | 0.50 | 1 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugolo, M.; Mascoloti Spréa, R.; Dias, M.I.; Pires, T.C.S.P.; Añibarro-Ortega, M.; Barroetaveña, C.; Caleja, C.; Barros, L. Nutritional Composition and Bioactive Properties of Wild Edible Mushrooms from Native Nothofagus Patagonian Forests. Foods 2022, 11, 3516. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11213516
Rugolo M, Mascoloti Spréa R, Dias MI, Pires TCSP, Añibarro-Ortega M, Barroetaveña C, Caleja C, Barros L. Nutritional Composition and Bioactive Properties of Wild Edible Mushrooms from Native Nothofagus Patagonian Forests. Foods. 2022; 11(21):3516. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11213516
Chicago/Turabian StyleRugolo, Maximiliano, Rafael Mascoloti Spréa, Maria Inês Dias, Tânia C. S. P. Pires, Mikel Añibarro-Ortega, Carolina Barroetaveña, Cristina Caleja, and Lillian Barros. 2022. "Nutritional Composition and Bioactive Properties of Wild Edible Mushrooms from Native Nothofagus Patagonian Forests" Foods 11, no. 21: 3516. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11213516