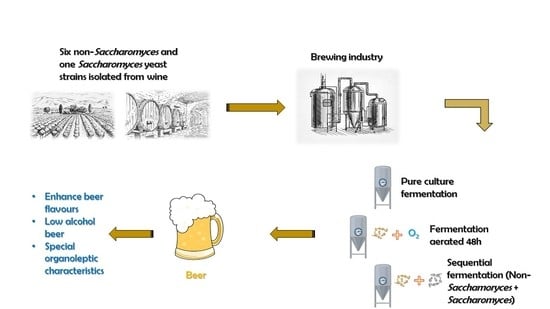
Non-Conventional Yeast: Behavior under Pure Culture, Sequential and Aeration Conditions in Beer Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Wort
2.2. Lab-Scale Screening
2.3. Pilot Plant Scale: 7 L
2.4. Beer Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Screening
3.2. Pilot Plant Fermentation: 7 L
3.3. Volatile Components Analyzed in 7 L
3.4. Principal Components Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conway, J. Global Beer Production 1998–2021. Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/270275/worldwide-beer-production/ (accessed on 13 September 2022).
- Bellut, K.; Arendt, E.K. Chance and Challenge: Non-Saccharomyces Yeasts in Nonalcoholic and Low Alcohol Beer Brewing—A Review. J. Am. Soc. Brew. Chem. 2019, 77, 77–91. [Google Scholar] [CrossRef]
- Cubillos, F.A.; Gibson, B.; Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J. Bioprospecting for Brewers: Exploiting Natural Diversity for Naturally Diverse Beers. Yeast 2019, 36, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Hittinger, C.T.; Steele, J.L.; Ryder, D.S. Diverse Yeasts for Diverse Fermented Beverages and Foods. Curr. Opin. Biotechnol. 2018, 49, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Verstrepen, K.J. Taming Wild Yeast: Potential of Conventional and Nonconventional Yeasts in Industrial Fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Methner, Y.; Hutzler, M.; Matoulková, D.; Jacob, F.; Michel, M. Screening for the Brewing Ability of Different Non-Saccharomyces Yeasts. Fermentation 2019, 5, 101. [Google Scholar] [CrossRef] [Green Version]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora Delbrueckii in the Brewing Process: A New Approach to Enhance Bioflavour and to Reduce Ethanol Content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef]
- Callejo, M.J.; García Navas, J.J.; Alba, R.; Escott, C.; Loira, I.; González, M.C.; Morata, A. Wort Fermentation and Beer Conditioning with Selected Non-Saccharomyces Yeasts in Craft Beers. Eur. Food Res. Technol. 2019, 245, 1229–1238. [Google Scholar] [CrossRef]
- Michel, M.; Kopecká, J.; Meier-Dörnberg, T.; Zarnkow, M.; Jacob, F.; Hutzler, M. Screening for New Brewing Yeasts in the Non-Saccharomyces Sector with Torulaspora Delbrueckii as Model. Yeast 2016, 33, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Einfalt, D. Barley-Sorghum Craft Beer Production with Saccharomyces Cerevisiae, Torulaspora Delbrueckii and Metschnikowia Pulcherrima Yeast Strains. Eur. Food Res. Technol. 2021, 247, 385–393. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could Non-Saccharomyces Yeasts Contribute on Innovative Brewing Fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by Non-Conventional Yeasts in Sequential Beer Fermentations. Food Microbiol. 2018, 72, 55–66. [Google Scholar] [CrossRef]
- Bourbon-Melo, N.; Palma, M.; Rocha, M.P.; Ferreira, A.; Bronze, M.R.; Elias, H.; Sá-Correia, I. Use of Hanseniaspora Guilliermondii and Hanseniaspora Opuntiae to Enhance the Aromatic Profile of Beer in Mixed-Culture Fermentation with Saccharomyces Cerevisiae. Food Microbiol. 2021, 95, 103678. [Google Scholar] [CrossRef]
- Rodrigues, A.J.; Raimbourg, T.; Gonzalez, R.; Morales, P. Environmental Factors Influencing the Efficacy of Different Yeast Strains for Alcohol Level Reduction in Wine by Respiration. Lwt 2016, 65, 1038–1043. [Google Scholar] [CrossRef]
- Kirsop, B.H. OXYGEN IN BREWERY FERMENTATION. J. Inst. Brew. 1973, 80, 252–259. [Google Scholar] [CrossRef]
- Virkajärvi, I.; Lindborg, K.; Kronlöf, J.; Pajunen, E. Effects of Aeration on Flavor Compounds in Immobilized Primary Fermentation. Mon. Für Brauwiss 1999, 52, 9–28. [Google Scholar]
- Kucharczyk, K.; Tuszyński, T. The Effect of Wort Aeration on Fermentation, Maturation and Volatile Components of Beer Produced on an Industrial Scale. J. Inst. Brew. 2017, 123, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Postigo, V.; García, M.; Cabellos, J.M.; Arroyo, T. Wine Saccharomyces Yeasts for Beer Fermentation. Fermentation 2021, 7, 290. [Google Scholar] [CrossRef]
- Postigo, V.; Sánchez, A.; Cabellos, J.M.; Arroyo, T. New Approaches for the Fermentation of Beer: Non-Saccharomyces Yeasts from Wine. Fermentation 2022, 8, 280. [Google Scholar] [CrossRef]
- Vaudano, E.; Garcia-Moruno, E. Discrimination of Saccharomyces Cerevisiae Wine Strains Using Microsatellite Multiplex PCR and Band Pattern Analysis. Food Microbiol. 2008, 25, 56–64. [Google Scholar] [CrossRef]
- Montrocher, R.; Verner, M.C.; Briolay, J.; Gautier, C.; Marmeisse, R. Phylogenetic Analysis of the Saccharomyces Cerevisiae Group Based on Polymorphisms of RDNA Spacer Sequences. Int. J. Syst. Bacteriol. 1998, 48, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of Yeasts by RFLP Analysis of the 5.8S RRNA Gene and the Two Ribosomal Internal Transcribed Spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef] [PubMed]
- European Brewery Convention Analytica-EBC. Section 8 Fermentable Carbohydrates in Wort by HPLC Method 8.7. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2004. [Google Scholar]
- European Brewery Convention Analytica-EBC. Section 9 Dimethyl Sulphide and Other Lower Boiling Point Volatile Compounds in Beer by Gas Chromatography 9.39. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2000. [Google Scholar]
- Lachance, M.A. Metschnikowia Kamienski (1899); Elsevier: Amsterdam, The Netherlands, 2011; Volume 2, ISBN 9780444521491. [Google Scholar]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; De Schutter, D.; Daenen, L.; Lynch, K.; Zannini, E.; Arendt, E. Application of Non-Saccharomyces Yeasts Isolated from Kombucha in the Production of Alcohol-Free Beer. Fermentation 2018, 4, 66. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.H.; Hsu, W.H.; Lee, F.L.; Liao, C.C. The Isolation and Identification of Microbes from a Fermented Tea Beverage, Haipao, and Their Interactions during Haipao Fermentation. Food Microbiol. 1996, 13, 407–415. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Zyła, K.; Tuszyński, T. Simultaneous Optimization of Acetaldehyde and DMS Concentrations for Better Sensory Quality of Beer Fermented on an Industrial Scale. Foods 2020, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M. Flavor Chemistry of Beer: Part II: Flavor and Threshold of 239 Aroma Volatiles. Master Brew. Assoc. Am. Tech. Q. 1975, 12, 151–168. [Google Scholar]
- Eßlinger, H.M. Handbook of Brewing Processes Technology Markets-Wiley; Wiley: Weinheim, Germany, 2009; ISBN 9783527314065. [Google Scholar]
- Anness, B.J.; Bamforth, C.W.; Wainwright, T. The Measurement of Dimethyl Sulphoxide in Barley and Malt and Its Reduction To Dimethyl Sulphide By Yeast. J. Inst. Brew. 1979, 85, 346–349. [Google Scholar] [CrossRef]
- White, F.H.; Wainwright, T. The Presence of Two Dimethyl Sulphide Precursors. J. Inst. Brew. 1977, 83, 224–230. [Google Scholar] [CrossRef]
- Baldus, M.; Klie, R.; Biermann, M.; Kreuschner, P.; Hutzler, M.; Methner, F.J. On the Behaviour of Dimethyl Sulfoxide in the Brewing Process and Its Role as Dimethyl Sulfide Precursor in Beer. BrewingScience 2018, 71, 1–11. [Google Scholar] [CrossRef]
- Anness, B.J.; Bamforth, C.W. Dimethyl sulphide—A review. J. Inst. Brew. 1982, 88, 244–252. [Google Scholar] [CrossRef]
- Harrison, G.A.F. The flavour of beer—A review*. J. Inst. Brew. 1970, 76, 486–495. [Google Scholar] [CrossRef]
- Postel, W.; Drawert, F. Gas Chromatographic Determination of Volatile Components of Fermented Beverages. II. The Content of Volatile Components in Beer (Ger). Chem. Mikrobiol. Technol. Leb. 1972, 1, 169–182. [Google Scholar]
- Budroni, M.; Zara, G.; Ciani, M.; Comitini, F. Saccharomyces and Non-Saccharomyces Starter Yeasts. In Brewing Technology; InTech: Rijeka, Croatia, 2017; pp. 81–100. [Google Scholar]
- Jakobsen, M.; Thorne, R.S.W. Oxygen Requirements of Brewing Strains of Saccharomyces Uvarum (Carlsbergensis)—Bottom Fermentation Yeast. J. Inst. Brew. 1980, 86, 284–287. [Google Scholar] [CrossRef]
- Verbelen, P.J.; Depraetere, S.A.; Winderickx, J.; Delvaux, F.R.; Delvaux, F. The Influence of Yeast Oxygenation Prior to Brewery Fermentation on Yeast Metabolism and the Oxidative Stress Response. FEMS Yeast Res. 2009, 9, 226–239. [Google Scholar] [CrossRef]
- Pearlstein, K.M. Pilot-Scale Studies on Extended Aeration at Fermentor Fill. J. Am. Soc. Brew. Chem. 1988, 46, 5–9. [Google Scholar] [CrossRef]
- Mortazavian, A.M.; Razavi, S.H.; Mousavi, S.M. The Effect of Saccharomyces Strain and Fermentation Conditions on Quality Prameters of Non-Alcoholic Beer. J. Paramed. Sci. 2014, 5, 109–114. [Google Scholar] [CrossRef]
- Ricketts, R.W.; Hough, J.S. Influence of Aeration on Production of Beer By Continuous Fermentation. J. Inst. Brew. 1961, 67, 29–32. [Google Scholar] [CrossRef]
- Fukuhara, H. The Kluyver Effect Revisited. FEMS Yeast Res. 2003, 3, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Sims, A.P.; Barnett, J.A. The Requirement of Oxygen for the Utilization of Maltose, Cellobiose and D-Galactose by Certain Anaerobically Fermenting Yeasts (Kluyver Effect). J. Gen. Microbiol. 1978, 106, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Lage, P.; Barbosa, C.; Mateus, B.; Vasconcelos, I.; Mendes-Faia, A.; Mendes-Ferreira, A.H. Guilliermondii Impacts Growth Kinetics and Metabolic Activity of S. Cerevisiae: The Role of Initial Nitrogen Concentration. Int. J. Food Microbiol. 2014, 172, 62–69. [Google Scholar] [CrossRef]
- Da Silva, G.A.; Augusto, F.; Poppi, R.J. Exploratory Analysis of the Volatile Profile of Beers by HS-SPME-GC. Food Chem. 2008, 111, 1057–1063. [Google Scholar] [CrossRef] [Green Version]
- Stewart, G. The Production of Secondary Metabolites with Flavour Potential during Brewing and Distilling Wort Fermentations. Fermentation 2017, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.; Caldeira, M.; Câmara, J.S. Development of a Dynamic Headspace Solid-Phase Microextraction Procedure Coupled to GC-QMSD for Evaluation the Chemical Profile in Alcoholic Beverages. Anal. Chim. Acta 2008, 609, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Siebert, K.J. Quantitative Structure-Activity Relationship Modeling of Alcohol, Ester, Aldehyde, and Ketone Flavor Thresholds in Beer from Molecular Features. J. Agric. Food Chem. 2004, 52, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The Soul of Beer’s Aroma—A Review of Flavour-Active Esters and Higher Alcohols Produced by the Brewing Yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrés-Iglesias, C.; Blanco, C.A.; García-Serna, J.; Pando, V.; Montero, O. Volatile Compound Profiling in Commercial Lager Regular Beers and Derived Alcohol-Free Beers After Dealcoholization by Vacuum Distillation. Food Anal. Methods 2016, 9, 3230–3241. [Google Scholar] [CrossRef]
- Liguori, L.; De Francesco, G.; Russo, P.; Perretti, G.; Albanese, D.; Di Matteo, M. Quality Attributes of Low-Alcohol Top-Fermented Beers Produced by Membrane Contactor. Food Bioprocess Technol. 2016, 9, 191–200. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and Non-Conventional Yeasts in Beer Production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Shimizu, H.; Shioya, S. Beer Volatile Compounds and Their Application to Low-Malt Beer Fermentation. J. Biosci. Bioeng. 2008, 106, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Brányik, T.; Vicente, A.A.; Cruz, J.M.M.; Teixeira, J.A. Continuous Primary Fermentation of Beer with Yeast Immobilized on Spent Grains—The Effect of Operational Conditions. J. Am. Soc. Brew. Chem. 2004, 62, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.L.; Margaritis, A.; Stewart, R.J. The Combined Effects of Oxygen Supply Strategy, Inoculum Size and Temperature Profile on Very-High-Gravity Beer Fermentation by Saccharomyces Cerevisiae. J. Inst. Brew. 2007, 113, 168–184. [Google Scholar] [CrossRef]
- Mauricio, J.C.; Moreno, J.; Zea, L.; Ortega, J.M.; Medina, M. The Effects of Grape Must Fermentation Conditions on Volatile Alcohols and Esters Formed by Saccharomyces Cerevisiae. J. Sci. Food Agric. 1997, 75, 155–160. [Google Scholar] [CrossRef]
- Delcour, J.A.; Dondeyne, P.; Trousdale, E.K.; Singleton, V.L. The Reactions Between Polyphenols and Aldehydes and the Influence of Acetaldehyde on Haze Formation in Beer. J. Inst. Brew. 1982, 88, 234–243. [Google Scholar] [CrossRef]
- Lodolo, E.J.; Kock, J.L.F.; Axcell, B.C.; Brooks, M. The Yeast Saccharomyces Cerevisiae—The Main Character in Beer Brewing. FEMS Yeast Res. 2008, 8, 1018–1036. [Google Scholar] [CrossRef]
- Anderson, R.J.; Howard, G.A. The Origin and Occurrence of Volatile Sulphur Compounds in British Ales and Lagers. J. Inst. Brew. 1974, 80, 357–370. [Google Scholar] [CrossRef]
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Methner, F.J.; Wagner, R.S.; Hutzler, M. Review: Pure Non-Saccharomyces Starter Cultures for Beer Fermentation with a Focus on Secondary Metabolites and Practical Applications. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Capece, A.; De Fusco, D.; Pietrafesa, R.; Siesto, G.; Romano, P. Performance of Wild Non-Conventional Yeasts in Fermentation of Wort Based on Different Malt Extracts to Select Novel Starters for Low-Alcohol Beers. Appl. Sci. 2021, 11, 801. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate Ester Formation in Wine by Mixed Cultures in Laboratory Fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Holt, S.; Miks, M.H.; De Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. The Molecular Biology of Fruity and Floral Aromas in Beer and Other Alcoholic Beverages. FEMS Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef] [Green Version]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-Vinyl and 4-Ethyl Derivatives from Hydroxycinnamic Acids: Occurrence of Volatile Phenolic Flavour Compounds in Beer and Distribution of Pad1-Activity among Brewing Yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L.; et al. Domestication and Divergence of Saccharomyces Cerevisiae Beer Yeasts. Cell 2016, 166, 1397–1410. [Google Scholar] [CrossRef]



| Yeast Strains | Glucose | Fructose | Sucrose | Maltose | Maltotriose | Ethanol % (v/v) | Final Apparent Extract % (m/m) | Apparent Attenuation (%) |
|---|---|---|---|---|---|---|---|---|
| CLI 101 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.33 ± 0.00 b | 6.56 ± 0.08 b | 2.02 ± 0.01 a | 1.16 ± 0.33 bc | 13.29 ± 0.28 cd | 20.59 |
| CLI 622 | 0.01 ± 0.00 b | 0.02 ± 0.00 b | 0.38 ± 0.01 a | 7.05 ± 0.02 a | 1.99 ± 0.01 ab | 1.07 ± 0.19 bc | 13.98 ± 0.12 b | 16.18 |
| CLI 691 | 0.01 ± 0.00 b | 0.02 ± 0.00 b | 0.38 ± 0.01 a | 7.00 ± 0.04 a | 1.98 ± 0.03 ab | 1.07 ± 0.19 bc | 13.91 ± 0.19 bc | 16.18 |
| CLI 895 | 0.01 ± 0.00 b | 0.02 ± 0.00 b | 0.01 ± 0.00 c | 7.07 ± 0.04 a | 2.05 ± 0.04 a | 1.24 ± 0.44 b | 13.11 ± 0.09 d | 22.06 |
| CLI 901 | 1.61 ± 0.05 a | 0.67 ± 0.01 a | 0.38 ± 0.01 a | 6.93 ± 0.04 a | 1.95 ± 0.01 b | 0.01 ± 0.00 c | 15.98 ± 0.21 a | 2.94 |
| CLI 1057 | 0.01 ± 0.00 b | 0.02 ± 0.00 b | 0.01 ± 0.00 c | 6.96 ± 0.13 a | 1.99 ± 0.01 ab | 1.26 ± 0.47 b | 13.12 ± 0.08 d | 22.06 |
| S-04 | 0.01 ± 0.00 b | 0.02 ± 0.00 b | 0.01 ± 0.00 c | 0.02 ± 0.00 c | 0.04 ± 0.00 c | 5.69 ± 0.23 a | 1.95 ± 0.01 e | 88.24 |
| Yeast Strains | Acetaldehyde (mg L−1) | DMS (μg L−1) | Acetone (mg L−1) | Total Higher Alcohols (mg L−1) | Total Esters (mg L−1) |
|---|---|---|---|---|---|
| CLI 101 | 13.05 ± 4.17 b | <17.00 b | 1.48 ± 0.02 | 156.55 ± 13.51 b | 7.43 ± 3.05 b |
| CLI 622 | 2.70 ± 0.28 b | <17.00 b | 1.12 ± 0.10 | 121.45 ± 5.30 bc | 2.17 ± 0.14 b |
| CLI 691 | 2.40 ± 0.14 b | 33.80 ± 10.61 a | 1.04 ± 0.09 | 143.40 ± 2.97 bc | 3.12 ± 0.21 b |
| CLI 895 | 24.45 ± 4.88 b | <17.00 b | 1.13 ± 0.29 | 143.55 ± 14.07 bc | 7.95 ± 1.70 b |
| CLI 901 | 0.45 ± 0.07 b | 17.00 ± 0.01 b | 1.02 ± 0.35 | 5.00 ± 0.00 d | 0.55 ± 0.00 b |
| CLI 1057 | >30 a | 17.35 ± 0.49 b | 0.96 ± 0.06 | 109.00 ± 11.59 c | 3.99 ± 0.32 b |
| S-04 | >30 a | <17.00 b | 0.97 ± 0.08 | 304.05 ± 15.34 a | 80.37 ± 17.65 a |
| Compounds | CLI 101 | CLI 512 | CLI 622 | CLI 895 | CLI 1057 | S-04 | Experiment |
|---|---|---|---|---|---|---|---|
| Acetaldehyde | 0.64 ± 0.21 | 4.04 ± 4.76 | 8.45 ± 10.94 | 1.55 ± 0.10 | 0.71 ± 0.06 | 0.93 ± 0.04 | A |
| 4.19 ± 1.90 | 1.26 ± 0.34 | 1.59 ± 0.33 | 2.55 ± 0.15 | 1.78 ± 0.89 | B | ||
| 1.11 ± 0.04 | 0.89± 0.05 | 0.95 ± 0.30 | C | ||||
| DMS | 13.48 ± 2.87 efg | 1.94 ± 0.47 gh | 19.07 ± 3.51 def | 27.26 ± 4.02 cd | 50.84 ± 1.70 ab | 25.11 ± 2.97 de | A |
| (μg L−1) | nd | nd | 26.12 ± 5.28 cde | 2.16 ± 0.77 cd | 9.54 ± 1.99 fgh | B | |
| 38.35 ± 0.53 bc | 62.66 ± 8.43 a | 28.14 ± 0.69 gh | C | ||||
| Acetone | 0.30 ± 0.23 | 0.39 ± 0.06 | 0.28 ± 0.03 | 0.23 ± 0.00 | 0.22 ± 0.25 | 0.30 ± 0.10 | A |
| 0.06 ± 0.01 | 0.29 ± 0.09 | 0.15 ± 0.04 | 0.14 ± 0.05 | 0.10 ± 0.02 | B | ||
| 0.26 ± 0.04 | 0.27 ± 0.03 | 0.12 ± 0.09 | C | ||||
| Diacetyl | 478.89 ± 146.30 a | 56.43 ±0.71 b | 21.35 ± 1.14 b | 57.16 ± 2.53 b | 96.46 ± 53.63 b | 5.57 ± 2.53 b | A |
| 29.25 ± 14.89 b | 49.88 ± 2.20 b | 17.80 ± 10.50 b | 22.40 ± 9.28 b | 37.81 ± 12.57 b | B | ||
| 35.74 ± 18.32 b | 18.03 ± 4.84 b | 55.69 ± 5.80 b | C | ||||
| 2,3- | 4.96 ± 0.59 c | 28.30 ± 6.40 abc | 4.41 ± 0.26 c | 5.52 ± 2.64 bc | 40.65 ± 24.37 a | 0.69 ± 0.12 c | A |
| pentanedione | 3.47 ± 2.98 c | 37.52 ± 13.81 ab | 2.24 ± 1.18 c | 1.60 ± 0.42 c | 7.71 ± 3.20 bc | B | |
| 8.08 ± 5.34 bc | 5.03 ± 1.13 c | 17.74 ± 4.51 ab | C | ||||
| Ethyl formate | 0.01 ± 0.00 d | 0.01 ± 0.02 d | nd | 0.02 ± 0.01 cd | 0.04 ± 0.01 cd | 0.09 ±0.01 ab | A |
| 0.01 ± 0.01 d | nd | 0.01 ± 0.00 b | 0.01 ± 0.00 d | 0.10 ± 0.03 a | B | ||
| 0.06 ± 0.01 abc | 0.06 ± 0.00 bc | 0.06 ± 0.00 bc | C | ||||
| Ethyl acetate | 1.93 ± 0.66 d | 10.70 ± 3.03 d | 2.51 ± 0.07 d | 4.79 ± 0.17 d | 5.69 ± 0.22 d | 78.97 ± 10.57 ab | A |
| 3.86 ± 0.12 d | 50.58 ± 7.55 c | 1.28 ± 0.20 d | 6.06 ± 1.90 d | 15.23 ± 4.04 d | B | ||
| 96.37 ± 10.47 a | 71.33 ± 6.89 b | 50.21 ± 0.51 c | C | ||||
| Methanol | 3.31 ± 0.24 abcd | 1.90 ± 0.36 cd | 2.62 ± 0.03 bcd | 2.85 ± 0.11 bcd | 3.12 ± 0.49 bcd | 4.87 ± 0.05 a | A |
| 1.85 ± 0.26 d | 1.76 ± 0.15 d | 2.43 ± 0.65 bcd | 2.55 ± 0.98 bcd | 2.70 ± 0.14 bcd | B | ||
| 3.70 ± 0.13 ab | 3.51 ± 0.48 abc | 2.92 ± 0.36 bcd | C | ||||
| Ethyl | 0.00 ± 0.00 d | 0.14 ± 0.12 cd | 0.02 ± 0.00 e | 0.13 ± 0.01 cd | 0.01 ± 0.01 d | 1.18 ± 0.07 a | A |
| propionate | 0.02 ± 0.01 d | 0.08 ± 0.08 cd | 0.02 ± 0.01 d | 0.08 ± 0.00 cd | 0.17 ± 0.01 cd | B | |
| 0.26 ± 0.10 c | 0.21 ± 0.01 cd | 0.54 ± 0.08 b | C | ||||
| Propanol | 2.04 ± 0.20 d | 5.73 ± 1.83 cd | 6.45 ± 2.55 cd | 7.45 ± 0.15 cd | 13.14 ± 0.66 c | 44.36 ± 2.37 ab | A |
| 6.18 ± 0.66 cd | 5.28 ± 3.61 cd | 10.07 ± 1.90 cd | 11.56 ± 1.66 cd | 40.63 ± 2.38 ab | B | ||
| 50.15 ± 6.42 a | 37.96 ± 3.45 b | 49.82 ± 2.28 a | C | ||||
| Isobutanol | 20.15 ± 1.42 ef | 8.43 ± 2.09 hi | 8.57 ± 2.34 ghi | 4.48 ± 0.37 i | 18.91 ± 1.67 efgh | 52.73 ± 1.44 b | A |
| 19.41 ± 1.65 efg | 11.18 ± 4.03 fghi | 21.80 ± 4.67 ef | 23.54 ± 3.91 e | 93.43 ± 0.34 a | B | ||
| 44.57 ± 3.01 bc | 24.50 ± 2.74 de | 35.39 ± 3.96 cd | C | ||||
| Isoamyl | 0.01 ± 0.00 e | 0.92 ± 0.02 e | 0.01 ± 0.01 e | 0.01 ± 0.00 e | 2.46 ± 0.14 cd | 9.55 ± 1.23 a | A |
| acetate | 0.01 ± 0.00 e | 0.15 ± 0.21 e | 0.14 ± 0.04 e | 0.03 ± 0.01 e | 0.92 ± 0.09 de | B | |
| 5.87 ± 1.45 b | 5.01 ± 0.66 b | 4.27 ± 0.06 bc | C | ||||
| Amyl alcohols | 29.71 ± 1.16 ef | 16.63 ± 4.29 f | 16.53 ± 4.71 f | 39.40 ± 2.43 e | 70.19 ± 2.40 d | 120.47 ± 1.79 b | A |
| 31.85 ± 1.98 ef | 31.51 ± 1.40 ef | 48.92 ± 6.92 e | 89.55 ± 11.59 cd | 201.41 ± 3.14 a | B | ||
| 110.01 ± 0.93 c | 70.23 ± 5.46 d | 90.20 ± 7.70 cd | C | ||||
| Ethyl caproate | 0.00 ± 0.00 e | 0.01 ± 0.01 e | 0.00 ± 0.00 e | 0.01 ± 0.00 e | 0.12 ± 0.03 cd | 0.32 ± 0.03 a | A |
| nd | 0.00 ± 0.00 e | 0.00 ± 0.00 e | nd | 0.06 ± 0.00 de | B | ||
| 0.22 ± 0.02 b | 0.17 ± 0.01 bc | 0.23 ± 0.04 b | C | ||||
| 4VG | 0.23 ± 0.02 b | 0.16 ± 0.02 b | 0.20 ± 0.04 b | 0.20 ± 0.03 b | 1.65 ± 0.95 a | 0.18 ± 0.02 b | A |
| 0.18 ± 0.01 b | 0.16 ± 0.02 b | 0.20 ± 0.05 b | 0.21 ± 0.05 b | 1.86 ± 0.00 a | B | ||
| 0.16 ± 0.02 b | 0.16 ± 0.01 b | 0.15 ± 0.00 b | C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postigo, V.; O’Sullivan, T.; Elink Schuurman, T.; Arroyo, T. Non-Conventional Yeast: Behavior under Pure Culture, Sequential and Aeration Conditions in Beer Fermentation. Foods 2022, 11, 3717. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11223717
Postigo V, O’Sullivan T, Elink Schuurman T, Arroyo T. Non-Conventional Yeast: Behavior under Pure Culture, Sequential and Aeration Conditions in Beer Fermentation. Foods. 2022; 11(22):3717. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11223717
Chicago/Turabian StylePostigo, Vanesa, Tadhg O’Sullivan, Tom Elink Schuurman, and Teresa Arroyo. 2022. "Non-Conventional Yeast: Behavior under Pure Culture, Sequential and Aeration Conditions in Beer Fermentation" Foods 11, no. 22: 3717. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11223717