Effect of Anti-Obesity and Antioxidant Activity through the Additional Consumption of Peel from ‘Fuji’ Pre-Washed Apple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Preparation and Water Contents Calculation
2.2. Antioxidant Assays and Antioxidant Compounds
2.2.1. Sample Extraction for Antioxidant Assays and Compounds
2.2.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.2.3. Ferric Reducing Antioxidant Power Assay (FRAP)
2.2.4. Total Flavonoid Content Assay (TFC)
2.2.5. Total Polyphenol Content Assay (TPC)
2.3. Triterpenoid Determination
2.3.1. Triterpenoid Extraction and Concentrated Extract Sample Preparation
2.3.2. Sample Derivatization for Quantification
2.4. Cell Culture
2.4.1. Cell Plate Preparation
2.4.2. WST-1 Assay
2.4.3. Oil-Red O Assay
2.4.4. Cell Lipid and Fatty Acid Quantification
2.5. Statistical Analysis
3. Results and Discussion
3.1. Plant Material Preparation and Water Contents Calculation
3.2. Total Antioxidant Activity and Antioxidant Compounds Determination
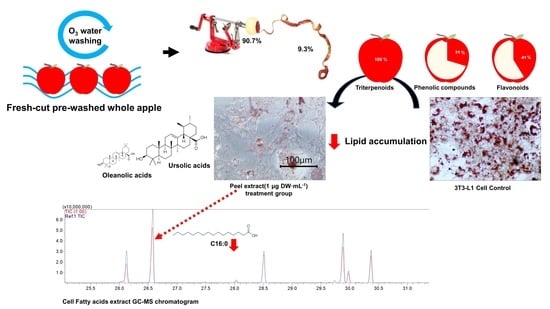
3.3. Effect of Apple Containing Triterpenoids on Cellular Lipid Droplets
3.4. 3T3-L1 Cell Lipid and Fatty Acid Suppression Effect by Different Apple Tissue Extracts
3.5. 3T3-L1 Cellular Fatty Acid Determination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Obesity and overweigh. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 13 January 2022).
- Meskin, M.S.; Bidlack, W.R.; Davies, A.; Omaye, S.T. Phytochemicals in Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–221. [Google Scholar]
- Hurt, R.T.; Kulisek, C.; Buchanan, L.A.; McClave, S.A. The obesity epidemic: Challenges, health initiatives, and implications for gastroenterologists. Gastroenterol. Hepatol. 2010, 6, 780–792. [Google Scholar]
- Martins, A.; Morgado, S.; Morgado, M. Anti-obesity drugs currently used and new compounds in clinical development. World J. Meta Anal. 2014, 2, 135–153. [Google Scholar] [CrossRef]
- Patti, A.M.; Al-Rasadi, K.; Giglio, R.V.; Nikolic, D.; Mannina, C.; Castellino, G.; Chianetta, R.; Banach, M.; Cicero, A.F.G.; Lippi, G.; et al. Natural approaches in metabolic syndrome management. Arch. Med. Sci. 2018, 14, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Lee, S.R.; Heo, J.-W.; No, M.-H.; Rhee, B.D.; Ko, K.S.; Kwak, H.-B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharm. 2018, 22, 235–248. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). Promoting Fruit and Vegetable Consumption. 2019. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/activities/technical-support-to-member-states/promoting-fruit-and-vegetable-consumption (accessed on 13 January 2022).
- Krebs-Smith, S.M.; Guenther, P.M.; Subar, A.F.; Kirkpatrick, S.I.; Dodd, K.W. Americans do not meet federal dietary recommendations. J. Nutr. 2010, 140, 1832–1838. [Google Scholar] [CrossRef]
- National Health Statistics Associated by Disease Control and Prevention in South Korea. Proportion with Adequate Intake of Vegetables and Fruits. 2018. Available online: https://www.kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&act=view&list_no=140377 (accessed on 13 January 2022).
- Rojas-Graü, M.; Garner, E.; Martin-Belloso, O. The fresh-cut fruit and vegetables industry: Current situation and market trends. In Advances in Fresh-Cut Fruits and Vegetables Processing; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–12. [Google Scholar]
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef]
- Kim, J.-G.; Luo, Y.; Lim, C.-I. Effect of Ozonated Water and Chlorine Water Wash on the Quality and Microbial De-contamination of Fresh-cut Carrot Shreds. Korean J. Food Preserv. 2007, 14, 54–60. [Google Scholar]
- Wang, S.; Wang, J.; Wang, T.; Li, C.; Wu, Z. Effects of ozone treatment on pesticide residues in food: A review. Int. J. Food Sci. Technol. 2019, 54, 301–312. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.E.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Morrison, S.; McGee, S.L. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte 2015, 4, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.-M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Rastqar, A.; Keshvari, M. Weight Loss Associated with Consumption of Apples: A Review. J. Am. Coll. Nutr. 2018, 37, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, M.; Wolfrum, C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 2014, 3, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.; Nogueira, R.; Martins, M.; Valladao, D.; Pires, E. The oven-drying method for determination of water content in Brazil nut. Biosci. J. 2018, 34, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Ku, K.M.; Kang, Y.H. Antioxidant and Quinone Reductase Inductive Activities of Various Organs of Pepper. J. Appl. Biol. Chem. 2010, 53, 31–36. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Jemmali, Z.; Chartier, A.; Dufresne, C.; Elfakir, C. Optimization of the derivatization protocol of pentacyclic triterpenes prior to their gas chromatography–mass spectrometry analysis in plant extracts. Talanta 2016, 147, 35–43. [Google Scholar] [CrossRef]
- Ramírez-Zacarías, J.L.; Castro-Muñozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with oil red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Parrilla, E.; Rosa, L.A.D.L.; Torres-Rivas, F.; Rodrigo-Garcia, J.; González-Aguilar, G.A. Complexation of Apple Antioxidants: Chlorogenic Acid, Quercetin and Rutin by β-Cyclodextrin (β-CD). J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 121–129. [Google Scholar] [CrossRef]
- Zhang, G.; Ji, B.; Chen, G. Antioxidant Activities and Phenolic Composition of Apple Peel, Core and Flesh Extracts on Selected Apple Cultivars. Adv. Mater. Res. 2012, 554–556, 1103–1109. [Google Scholar] [CrossRef]
- Kim, I.; Ku, K.-H.; Jeong, M.-C.; Kim, S.S.; Mitchell, A.E.; Lee, J. A comparison of the chemical composition and antioxidant activity of several new early- to mid-season apple cultivars for a warmer climate with traditional cultivars. J. Sci. Food Agric. 2019, 99, 6655. [Google Scholar] [CrossRef]
- Kjøllesdal, M.; Htet, A.S.; Stigum, H.; Hla, N.Y.; Hlaing, H.H.; Khaine, E.K.; Khaing, W.; Khant, A.K.; Khin, N.O.K.; Mauk, K.K.A.; et al. Consumption of fruits and vegetables and associations with risk factors for non-communicable diseases in the Yangon region of Myanmar: A cross-sectional study. BMJ Open 2016, 6, e011649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, P.; Lajous, M.; MacDonald, C.-J.; Fagherazzi, G.; Bonnet, F.; Boutron-Ruault, M.-C. High dietary total antioxidant capacity is associated with a reduced risk of hypertension in French women. Nutr. J. 2019, 18, 31. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, P.; Li, S.; Shah, N.P. Antioxidant, Antibacterial, and Antiproliferative Activities of Free and Bound Phenolics from Peel and Flesh of Fuji Apple. J. Food Sci. 2016, 81, M1735–M1742. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Torralba, A.; Guerra-Hernández, E.J.; García-Villanova, B. Antioxidant capacity, polyphenol content and contribution to dietary intake of 52 fruits sold in Spain. CyTA-J. Food 2018, 16, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Liu, R.H. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. J. Agric. Food Chem. 2007, 55, 4366–4370. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Geană, E.-I.; Ciucure, C.T.; Ionete, R.E.; Ciocârlan, A.; Aricu, A.; Ficai, A.; Andronescu, E. Profiling of Phenolic Compounds and Triterpene Acids of Twelve Apple (Malus domestica Borkh). Cultivars. Foods 2021, 10, 267. [Google Scholar] [CrossRef]
- Podsędek, A.; Zakłos-Szyda, M.; Polka, D.; Sosnowska, D. Effects of Viburnum opulus fruit extracts on adipogenesis of 3T3-L1 cells and lipase activity. J. Funct. Foods 2020, 73, 104111. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Elmore, C.J.; Bongers, K.S.; Ebert, S.M.; Fox, D.K.; Dyle, M.C.; Bullard, S.A.; Adams, C.M. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS ONE 2012, 7, e39332. [Google Scholar] [CrossRef] [Green Version]
- Katashima, C.K.; Silva, V.R.; Gomes, T.L.; Pichard, C.; Pimentel, G.D. Ursolic acid and mechanisms of actions on adipose and muscle tissue: A systematic review. Obes. Rev. 2017, 18, 700–711. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Zhao, T.; Wang, Y.; Sun, C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS one 2013, 8, e70135. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Saltiel, A.R. From overnutrition to liver injury: AMP-activated protein kinase in nonalcoholic fatty liver diseases. J. Biol. Chem. 2020, 295, 12279–12289. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, Y.; Liu, Y.; Liu, D.; Liu, Y.; Guo, Y.; Wu, Z.; Li, H.; Wang, H. Ursolic acid alleviates lipid accumulation by activating the AMPK signaling pathway in vivo and in vitro. J. Food Sci. 2020, 85, 3998–4008. [Google Scholar] [CrossRef] [PubMed]
- Butkeviciute, A.; Viskelis, J.; Liaudanskas, M.; Viskelis, P.; Bobinas, C.; Janulis, V. Variation of Triterpenes in Apples Stored in a Controlled Atmosphere. Molecules 2021, 26, 3639. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y. Ursolic acid, a promising dietary bioactive compound of anti-obesity (1045.40). FASEB J. 2014, 28, 10451040. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, W.; Ma, X.-F.; Ding, W. Evaluation of inhibition of fatty acid synthase by ursolic acid: Positive cooperation mechanism. Biochem. Biophys. Res. Commun. 2010, 392, 386–390. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Lisai, S.; Sirigu, A.; Piras, A.; Collu, M.; Batetta, B.; Gambelli, L.; Banni, S. Dietary triacylglycerols with palmitic acid in the sn-2 position modulate levels of N-acylethanolamides in rat tissues. PLoS ONE 2015, 10, e0120424. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liao, X.; Meng, F.; Wang, Y.; Sun, Z.; Guo, F.; Li, X.; Meng, M.; Li, Y.; Sun, C. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS ONE 2014, 9, e86724. [Google Scholar] [CrossRef] [Green Version]
- Morrison, R.F.; Farmer, S.R. Hormonal Signaling and Transcriptional Control of Adipocyte Differentiation. J. Nutr. 2000, 130, 3116S–3121S. [Google Scholar] [CrossRef]





| Pre-Washed Apple (100%) z | ||
|---|---|---|
| Flesh (90.7%) | Peel (9.3%) | |
| Fresh weight (g) | 212.4 ± 8.2 | 21.7 ± 10.2 |
| Dry weight (g) | 31.0 ± 1.2 | 4.3 ± 2.0 |
| Moist content (%) | 85.4 ± 0.7 | 80.0 ± 0.7 |
| Fatty Acids Concentration (μg·105 Cells−1) | 1 mg DW·mL−1 | 5 μM | |||
|---|---|---|---|---|---|
| Control | Whole Apple (Peel + Flesh) | Flesh | Peel | Ursolic Acid | |
| C16:0 | 11.97 ± 0.60a | 10.61 ± 1.52ab | 12.15 ± 1.05a | 8.09 ± 1.40b | 7.53 ± 2.08b |
| C18:0 | 5.49 ± 0.67a | 4.84 ± 1.33a | 5.17 ± 1.25a | 3.25 ± 0.72a | 3.48 ± 0.68a |
| C18:1n-9 (c) | 3.88 ± 0.24a | 3.13 ± 0.83b | 3.37 ± 0.74a | 2.07 ± 0.48b | 2.41 ± 0.49a |
| C16:1 | 2.64 ± 0.20a | 1.86 ± 0.29bc | 2.17 ± 0.18ab | 1.34 ± 0.16d | 1.47 ± 0.28cd |
| C14:0 | 1.35 ± 0.12a | 0.97 ± 0.16ab | 1.13 ± 0.17a | 0.70 ± 0.12b | 0.71 ± 0.18b |
| C16:1/C16:0 | 0.22 ± 0.01a | 0.18 ± 0.01a | 0.18 ± 0.00a | 0.17 ± 0.02b | 0.20 ± 0.03a |
| C18:1/C18:0 | 0.71 ± 0.05a | 0.65 ± 0.01a | 0.65 ± 0.02a | 0.64 ± 0.04b | 0.69 ± 0.00a |
| MUFA/SFA | 0.40 ± 0.00a | 0.35 ± 0.03a | 0.35 ± 0.03a | 0.33 ± 0.05a | 0.39 ± 0.04a |
| Total SFAz | 19.08 ± 1.19a | 16.66 ± 2.97ab | 18.70 ± 2.27a | 12.20 ± 2.00c | 11.90 ± 2.97bc |
| Total MUFAy | 7.59 ± 0.50a | 5.91 ± 1.43a | 6.53 ± 1.12a | 4.05 ± 0.76b | 4.58 ± 0.87b |
| Total PFAx | 1.01 ± 0.16a | 0.89 ± 0.33a | 0.92 ± 0.33a | 0.57 ± 0.19a | 0.70 ± 0.18a |
| Total FA | 27.77 ± 1.83a | 23.54 ± 4.74a | 26.23 ± 3.69a | 16.86 ± 2.73b | 17.25 ± 4.02b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, D.-Y.; Ku, K.-M. Effect of Anti-Obesity and Antioxidant Activity through the Additional Consumption of Peel from ‘Fuji’ Pre-Washed Apple. Foods 2022, 11, 497. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11040497
Ko D-Y, Ku K-M. Effect of Anti-Obesity and Antioxidant Activity through the Additional Consumption of Peel from ‘Fuji’ Pre-Washed Apple. Foods. 2022; 11(4):497. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11040497
Chicago/Turabian StyleKo, Da-Yeong, and Kang-Mo Ku. 2022. "Effect of Anti-Obesity and Antioxidant Activity through the Additional Consumption of Peel from ‘Fuji’ Pre-Washed Apple" Foods 11, no. 4: 497. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11040497