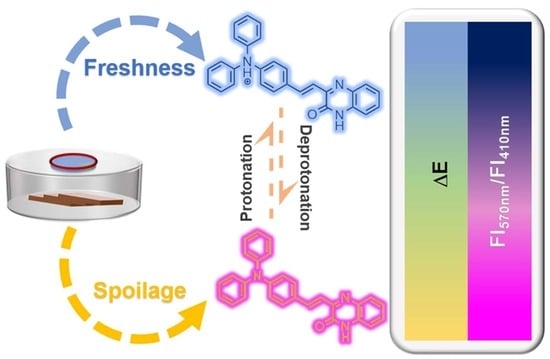
Ratiometric Monitoring of Biogenic Amines by a Simple Ammonia-Response Aiegen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of H+MQ-Loaded Paper Chip
2.3. Ammonia Response of H+MQ-Loaded Paper Chip
2.4. Stability Analysis of H+MQ-Loaded Paper Chips
2.5. Reusability of H+MQ-Loaded Paper Chip
2.6. Freshness Monitoring by Testing BAs with H+MQ-Loaded Paper Chip
3. Results and Discussion
3.1. Characterization and Photophysical Properties of MQ
3.2. Preparation and Characterization of the H+MQ-Loaded Paper Chip
3.3. Monitoring of Meat and Seafood Freshness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hallström, E.; Bergman, K.; Mifflin, K.; Parker, R.; Tyedmers, P.; Troell, M.; Ziegler, F. Combined climate and nutritional performance of seafoods. J. Clean. Prod. 2019, 230, 402–411. [Google Scholar] [CrossRef]
- Liao, W.; Wang, G.; Zhao, W.; Zhang, M.; Wu, Y.; Liu, X.; Li, K. Change in mercury speciation in seafood after cooking and gastrointestinal digestion. J. Hazard. Mater. 2019, 375, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.D.; Price, E.M.; Morgan, S.A.; Huws, S.A.; Shingfield, K.J. Can we improve the nutritional quality of meat? Proc. Nutr. Soc. 2017, 76, 603–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bank, M.S.; Metian, M.; Swarzenski, P.W. Defining seafood safety in the anthropocene. Environ. Sci. Technol. 2020, 54, 8506–8508. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Arthur, M.; Viglia, S.; Xue, J.; Meng, F.; Lombardi, G.V. Seafood-energy-water nexus: A study on resource use efficiency and the environmental impact of seafood consumption in China. J. Clean. Prod. 2020, 277, 124088. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, M.; Zheng, J.; Yang, S.; Yang, J. Effect of extra oxygen on Apostichopus japonicus life sustainability during commercial transport. Food Control 2021, 125, 108022. [Google Scholar] [CrossRef]
- Pannier, L.; Gardner, G.; Pearce, K.; McDonagh, M.; Ball, A.; Jacob, R.; Pethick, D. Associations of sire estimated breeding values and objective meat quality measurements with sensory scores in Australian lamb. Meat Sci. 2014, 96, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.K.; Zór, K.; Kostesha, N.; Alstrøm, T.S.; Heiskanen, A.; El-Tanahi, H.; Sharoba, A.; Papkovsky, D.; Larsen, J.; Khalaf, H.; et al. Development and validation of a colorimetric sensor array for fish spoilage monitoring. Food Control 2016, 60, 346–352. [Google Scholar] [CrossRef]
- Shafiei, R.; Mostaghim, T. Improving shelf life of calf fillet in refrigerated storage using edible coating based on chitosan/natamycin containing Spirulina platensis and Chlorella vulgaris microalgae. J. Food Meas. Charact. 2021, 16, 145–161. [Google Scholar] [CrossRef]
- Yang, S.; Pei, X.; Yang, D.; Zhang, H.; Chen, Q.; Chui, H.; Qiao, X.; Huang, Y.; Liu, Q. Microbial contamination in bulk ready-to-eat meat products of China in 2016. Food Control 2018, 91, 113–122. [Google Scholar] [CrossRef]
- Nache, M.; Hinrichs, J.; Scheier, R.; Schmidt, H.; Hitzmann, B. Prediction of the pH as indicator of porcine meat quality using Raman spectroscopy and metaheuristics. Chemom. Intell. Lab. Syst. 2016, 154, 45–51. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žurga, P.; Vahčić, N.; Pasković, I.; Banović, M.; Malenica Staver, M. Occurence of ochratoxin A and biogenic amines in Croatian commercial red wines. Foods 2019, 8, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, R.; Cardoso, C.; Pestana, C. Measurement of malondialdehyde in fish: A comparison study between HPLC methods and the traditional spectrophotometric test. Food Chem. 2009, 112, 1038–1045. [Google Scholar] [CrossRef]
- Huang, J.; Gan, N.; Lv, F.; Cao, Y.; Ou, C.; Tang, H. Environmentally friendly solid-phase microextraction coupled with gas chromatography and mass spectrometry for the determination of biogenic amines in fish samples. J. Sep. Sci. 2016, 39, 4384–4390. [Google Scholar] [CrossRef] [PubMed]
- Ščavničar, A.; Rogelj, I.; Kočar, D.; Köse, S.; Pompe, M. Determination of biogenic amines in cheese by ion chromatography with tandem mass spectrometry detection. J. AOAC Int. 2018, 101, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Adımcılar, V.; Öztekin, N.; Erim, F.B. A direct and sensitive analysis method for biogenic amines in dairy products by capillary electrophoresis coupled with contactless conductivity detection. Food Anal. Methods 2017, 11, 1374–1379. [Google Scholar] [CrossRef]
- Bibi, F.; Guillaume, C.; Gontard, N.; Sorli, B. Wheat gluten, a bio-polymer to monitor carbon dioxide in food packaging: Electric and dielectric characterization. Sens. Actuators B Chem. 2017, 250, 76–84. [Google Scholar] [CrossRef]
- Saetia, K.; Schnorr, J.M.; Mannarino, M.M.; Kim, S.Y.; Rutledge, G.C.; Swager, T.M.; Hammond, P.T. Spray-layer-by-layer carbon nanotube/electrospun fiber electrodes for flexible chemiresistive sensor applications. Adv. Funct. Mater. 2014, 24, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Andre, R.S.; Facure, M.H.; Mercante, L.A.; Correa, D.S. Electronic nose based on hybrid free-standing nanofibrous mats for meat spoilage monitoring. Sens. Actuators B Chem. 2022, 353, 131114. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, C.S.; Park, S.J.; Kim, J.; Seo, S.E.; An, J.E.; Ha, S.; Bae, J.; Phyo, S.; Lee, J.; et al. In-situ food spoilage monitoring using a wireless chemical receptor-conjugated graphene electronic nose. Biosens. Bioelectron. 2022, 200, 113908. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Yang, H.; Jeong, G.E.; Moon, D.; Kwon, O.S.; Phyo, S.; Lee, J.; Song, H.S.; Park, T.H.; Jang, J. Ultrasensitive, selective, and highly stable bioelectronic nose that detects the liquid and gaseous cadaverine. Anal. Chem. 2019, 91, 12181–12190. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Barat, J.M.; Escriche, I.; Garcia-Breijo, E.; Martínez-Máñez, R.; Soto, J. An electronic tongue for fish freshness analysis using a thick-film array of electrodes. Mikrochim. Acta 2008, 163, 121–129. [Google Scholar] [CrossRef]
- Sentellas, S.; Nuñez, O.; Saurina, J. Recent advances in the determination of biogenic amines in food samples by (U)HPLC. J. Agric. Food Chem. 2016, 64, 7667–7678. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, S.; Yeung, C.-C.; Li, T.; Lau, S.C.; Sun, Q.-J.; Li, L.-Y.; Li, J.H.; Lam, M.H.; Roy, V.A. Portable molecularly imprinted polymer-based platform for detection of histamine in aqueous solutions. J. Hazard. Mater. 2021, 410, 124609. [Google Scholar] [CrossRef]
- Umapathi, R.; Sonwal, S.; Lee, M.J.; Rani, G.M.; Lee, E.-S.; Jeon, T.-J.; Kang, S.-M.; Oh, M.-H.; Huh, Y.S. Colorimetric based on-site sensing strategies for the rapid detection of pesticides in agricultural foods: New horizons, perspectives, and challenges. Coord. Chem. Rev. 2021, 446, 214061. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Liu, R.; Chen, C.; Zeng, S.; Liu, Q.; Ding, D. Endoplasmic reticulum targeted AIE bioprobe as a highly efficient inducer of immunogenic cell death. Sci. China Chem. 2020, 63, 1428–1434. [Google Scholar] [CrossRef]
- Song, Y.; Zong, L.; Zhang, L.; Li, Z. To form AIE product with the target analyte: A new strategy for excellent fluorescent probes, and convenient detection of hydrazine in seconds with test strips. Sci. China Chem. 2017, 60, 1596–1601. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Lu, P.; Chen, S.; Lam, J.W.Y.; Wang, Z.; Liu, Y.; Kwok, H.S.; Ma, Y.; Tang, B.Z. Changing the behavior of chromophores from aggregation-caused quenching to aggregation-induced emission: Development of highly efficient light emitters in the solid state. Adv. Mater. 2010, 22, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Huang, X.; Chen, C.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. AIEgen for cancer discrimination. Mater. Sci. Eng. R Rep. 2021, 146, 100649. [Google Scholar] [CrossRef]
- Alam, P.; Leung, N.L.C.; Su, H.; Qiu, Z.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. A highly sensitive bimodal detection of amine vapours based on aggregation induced emission of 1,2-dihydroquinoxaline derivatives. Chem. Eur. J. 2017, 23, 14911–14917. [Google Scholar] [CrossRef]
- Gao, M.; Li, S.; Lin, Y.; Geng, Y.; Ling, X.; Wang, L.; Qin, A.; Tang, B.Z. Fluorescent light-up detection of amine vapors based on aggregation-induced emission. ACS Sens. 2015, 1, 179–184. [Google Scholar] [CrossRef]
- Hou, J.; Du, J.; Hou, Y.; Shi, P.; Liu, Y.; Duan, Y.; Han, T. Effect of substituent position on aggregation-induced emission, customized self-assembly, and amine detection of donor-acceptor isomers: Implication for meat spoilage monitoring. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, Q.; Zhang, R.; Zhao, Z.; Leng, Y.; Lam, J.W.Y.; Xiong, Y.; Tang, B.Z. AIEgens: An emerging fluorescent sensing tool to aid food safety and quality control. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2297–2329. [Google Scholar] [CrossRef]
- Huang, X.; He, Z.; Guo, D.; Liu, Y.; Song, J.; Yung, B.C.; Lin, L.; Yu, G.; Zhu, J.-J.; Xiong, Y.; et al. “Three-in-one” nanohybrids as synergistic nanoquenchers to enhance no-wash fluorescence biosensors for ratiometric detection of cancer biomarkers. Theranostics 2018, 8, 3461–3473. [Google Scholar] [CrossRef]
- Huang, X.; Song, J.; Yung, B.C.; Huang, X.; Xiong, Y.; Chen, X. Ratiometric optical nanoprobes enable accurate molecular detection and imaging. Chem. Soc. Rev. 2018, 47, 2873–2920. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, X.; Cao, F.; Wang, W.; Qian, G.; Zhang, J. Target-activated and ratiometric photochromic probe for “double-check” detection of toxic thiols in live cells. Sci. China Chem. 2019, 62, 1204–1212. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, H.; Chen, Y.; Chen, Z.; Zhao, M.; Zou, L.; Liu, Y.; Liu, Z.; Zhao, Q.; Guo, Z.; et al. A ratiometric fluorescent sensor for tracking Cu(I) fluctuation in endoplasmic reticulum. Sci. China Ser. B Chem. 2019, 62, 465–474. [Google Scholar] [CrossRef]
- Wu, S.; Min, H.; Shi, W.; Cheng, P. Multicenter metal–organic framework-based ratiometric fluorescent sensors. Adv. Mater. 2019, 32, 1805871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, H.; Li, F.; Chen, Y.; Kwok, R.T.K.; Huang, Y.; Zhang, J.; Hou, J.; Tang, B.Z. A ratiometric fluorescent probe based on AIEgen for detecting HClO in living cells. Chem. Commun. 2020, 56, 14613–14616. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N. Correspondence between CIELAB and CIELUV color differences. Color Res. Appl. 1977, 2, 178–182. [Google Scholar] [CrossRef]
- Caicedo, M.; Echeverry, C.A.; Guimarães, R.R.; Ortiz, A.; Araki, K.; Insuasty, B. Microwave assisted synthesis of a series of charge-transfer photosensitizers having quinoxaline-2(1H)-one as anchoring group onto TiO2 surface. J. Mol. Struct. 2017, 1133, 384–391. [Google Scholar] [CrossRef]
- Tu, Y.; Yu, Y.; Xiao, D.; Liu, J.; Zhao, Z.; Liu, Z.; Lam, J.W.Y.; Tang, B.Z. An intelligent AIEgen with nonmonotonic multiresponses to multistimuli. Adv. Sci. 2020, 7, 2001845. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhong, Z.; Ou, W.; Shi, H.; Alam, P.; Tang, B.Z.; Qin, J.; Tang, Y. Semi-quantitative evaluation of seafood spoilage using filter-paper strips loaded with an aggregation-induced emission luminogen. Food Chem. 2020, 327, 127056. [Google Scholar] [CrossRef]
- He, S.; Chen, Y.; Yang, X.; Gao, J.; Su, D.; Deng, J.; Tian, B. Determination of biogenic amines in Chub Mackerel from different storage methods. J. Food Sci. 2020, 85, 1699–1706. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Conte-Junior, C.; Cunha, F.L.; Marsico, E.T.; Mano, S.B.; Franco, R.M. Validation of an HPLC methodology for the identification and quantification of biogenic amines in chicken meat. Food Anal. Methods 2013, 6, 1024–1032. [Google Scholar] [CrossRef]
- Durlu-Özkaya, F.; Ayhan, K.; Vural, N. Biogenic amines produced by Enterobacteriaceae isolated from meat products. Meat Sci. 2001, 58, 163–166. [Google Scholar] [CrossRef]
- Hernández-Jover, T.; Izquierdo-Pulido, M.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Biogenic amine sources in cooked cured shoulder pork. J. Agric. Food Chem. 1996, 44, 3097–3101. [Google Scholar] [CrossRef]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food-existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapenna, A.; Dell’Aglio, M.; Palazzo, G.; Mallardi, A. “Naked” gold nanoparticles as colorimetric reporters for biogenic amine detection. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124903. [Google Scholar] [CrossRef]
- Diaz, Y.J.; Page, Z.A.; Knight, A.S.; Treat, N.J.; Hemmer, J.R.; Hawker, C.J.; Read de Alaniz, J. A versatile and highly selective colorimetric sensor for the detection of amines. Chem. Eur. J. 2017, 23, 3562–3566. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Zhang, Y.; Zhou, Y.; Liu, S.G.; Gao, W.; Shi, X. Portable functional hydrogels based on silver metallization for visual monitoring of fish freshness. Food Control 2021, 123, 107824. [Google Scholar] [CrossRef]
- Meng, Y.; Yuan, C.; Du, C.; Jia, K.; Liu, C.; Wang, K.-P.; Chen, S.; Hu, Z.-Q. A coumarin-based portable fluorescent probe for rapid turn-on detection of amine vapors. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120152. [Google Scholar] [CrossRef]
- Jin, Y.-J.; Kwak, G. Detection of biogenic amines using a nitrated conjugated polymer. Sens. Actuators B Chem. 2018, 271, 183–188. [Google Scholar] [CrossRef]
- Kang, S.; Wang, H.; Guo, M.; Zhang, L.; Chen, M.; Jiang, S.; Li, X.; Jiang, S. Ethylene-vinyl Alcohol Copolymer–Montmorillonite Multilayer Barrier Film Coated with Mulberry Anthocyanin for Freshness Monitoring. J. Agric. Food Chem. 2018, 66, 13268–13276. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Chen, X.; Chen, R.; Tu, Y.; Lu, T.; Guo, Y.; Guo, L.; Xiong, Y.; Huang, X.; Tang, B.Z. Ratiometric Monitoring of Biogenic Amines by a Simple Ammonia-Response Aiegen. Foods 2022, 11, 932. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11070932
Guo X, Chen X, Chen R, Tu Y, Lu T, Guo Y, Guo L, Xiong Y, Huang X, Tang BZ. Ratiometric Monitoring of Biogenic Amines by a Simple Ammonia-Response Aiegen. Foods. 2022; 11(7):932. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11070932
Chicago/Turabian StyleGuo, Xujing, Xirui Chen, Rui Chen, Yujie Tu, Tianying Lu, Yuqian Guo, Liang Guo, Yonghua Xiong, Xiaolin Huang, and Ben Zhong Tang. 2022. "Ratiometric Monitoring of Biogenic Amines by a Simple Ammonia-Response Aiegen" Foods 11, no. 7: 932. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11070932