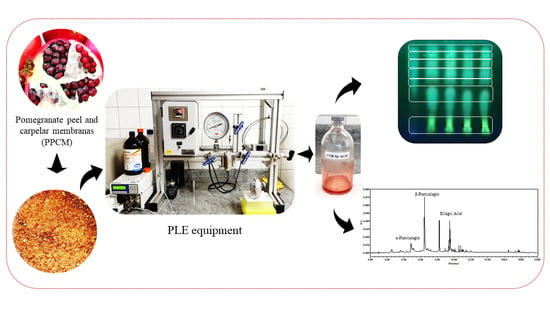
Phenolic Compounds Recovery from Pomegranate (Punica granatum L.) By-Products of Pressurized Liquid Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Reagents and Standard
2.3. PPCM Proximate Composition
2.4. Extraction Procedure by PLE
2.5. Extract Characterization
2.5.1. Global Extraction Yield
2.5.2. Qualitative Analysis of Phenolic Compounds
2.5.3. Quantitative Analysis of Punicalagins and Ellagic Acid
2.6. Statistical Analysis
3. Results
3.1. PPCM Proximate Composition
3.2. PLE Effects on the Global Extraction Yield
3.2.1. Pressure Effects on the Extraction Yield
3.2.2. Temperature Effects on the Extraction Yield
3.3. Phenolic Compounds Identification
3.4. Punicalagins and Ellagic Acid Content
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CEPALSTAT Food Waste (Tonnes) AG_FOOD_WST. Available online: https://cepalstat-prod.cepal.org/cepalstat/tabulador/ConsultaIntegrada.asp?IdAplicacion=45&idTema=1622&idIndicador=4363&idioma=i (accessed on 30 August 2021).
- FAO Food Loss and Waste Facts. Available online: http://www.fao.org/resources/infographics/infographics-details/en/c/317265 (accessed on 30 August 2021).
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic Compounds of Pomegranate Byproducts (Outer Skin, Mesocarp, Divider Membrane) and Their Antioxidant Activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: Application to different Italian varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Pomegranate. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier—Academic Press: Cambridge, MA, USA, 2019; pp. 181–216. ISBN 9780128141397. [Google Scholar]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Nowicka, P.; Munera-Picazo, S.; Hernández, F.; Carbonell-Barrachina, Á.A.; Wojdyło, A. Identification and quantification of major derivatives of ellagic acid and antioxidant properties of thinning and ripe Spanish pomegranates. J. Funct. Foods 2015, 12, 354–364. [Google Scholar] [CrossRef]
- Abid, M.; Yaich, H.; Cheikhrouhou, S.; Khemakhem, I.; Bouaziz, M.; Attia, H.; Ayadi, M.A. Antioxidant properties and phenolic profile characterization by LC–MS/MS of selected Tunisian pomegranate peels. J. Food Sci. Technol. 2017, 54, 2890–2901. [Google Scholar] [CrossRef]
- Orgil, O.; Schwartz, E.; Baruch, L.; Matityahu, I.; Mahajna, J.; Amir, R. The antioxidative and anti-proliferative potential of non-edible organs of the pomegranate fruit and tree. LWT-Food Sci. Technol. 2014, 58, 571–577. [Google Scholar] [CrossRef]
- Chaves, F.M.; Pavan, I.C.B.; da Silva, L.G.S.; de Freitas, L.B.; Rostagno, M.A.; Antunes, A.E.C.; Bezerra, R.M.N.; Simabuco, F.M. Pomegranate Juice and Peel Extracts are Able to Inhibit Proliferation, Migration and Colony Formation of Prostate Cancer Cell Lines and Modulate the Akt/mTOR/S6K Signaling Pathway. Plant Foods Hum. Nutr. 2020, 75, 54–62. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Licciardello, F.; Siracusa, L.; Muratore, G.; Hamdi, M.; Restuccia, C. Antimicrobial and antioxidant features of ‘Gabsiʼ pomegranate peel extracts. Ind. Crops Prod. 2018, 111, 345–352. [Google Scholar] [CrossRef]
- Li, Y.; Ye, T.; Yang, F.; Hu, M.; Liang, L.; He, H.; Li, Z.; Zeng, A.; Li, Y.; Yao, Y.; et al. Punica granatum (pomegranate) peel extract exerts potent antitumor and anti-metastasis activity in thyroid cancer. RSC Adv. 2016, 6, 84523–84535. [Google Scholar] [CrossRef]
- Abd-Allah, I.; Abd-Allah, I.; Rabie, M.; Rabie, M.; Sulieman, A.; Sulieman, A.; Mostfa, D.; Mostfa, D.; El-Badawi, A.; El-Badawi, A. Oxidative stability of edible oils via addition of pomegranate and orange peel extracts. Foods Raw Mater. 2018, 6, 413–420. [Google Scholar] [CrossRef]
- Çam, M.; Içyer, N.C.; Erdoǧan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT—Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef]
- Manna, K.; Mishra, S.; Saha, M.; Mahapatra, S.; Saha, C.; Yenge, G.; Gaikwad, N.; Pal, R.; Oulkar, D.; Banerjee, K.; et al. Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: Assessment of NF-κB and Nrf2 signaling system. Int. J. Nanomed. 2019, 14, 1753–1777. [Google Scholar] [CrossRef] [Green Version]
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process.—Process Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Rajha, H.N.; Mhanna, T.; El Kantar, S.; El Khoury, A.; Louka, N.; Maroun, R.G. Innovative process of polyphenol recovery from pomegranate peels by combining green deep eutectic solvents and a new infrared technology. LWT 2019, 111, 138–146. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2019, 101, 342–350. [Google Scholar] [CrossRef]
- Soquetta, M.B.; de Marsillac Terra, L.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CYTA—J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Sumere, B.R.; de Souza, M.C.; dos Santos, M.P.; Bezerra, R.M.N.; da Cunha, D.T.; Martinez, J.; Rostagno, M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162. [Google Scholar] [CrossRef]
- Turrini, F.; Zunin, P.; Catena, S.; Villa, C.; Alfei, S.; Boggia, R. Traditional or hydro-diffusion and gravity microwave coupled with ultrasound as green technologies for the valorization of pomegranate external peels. Food Bioprod. Process. 2019, 117, 30–37. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. ISBN 9780128169117. [Google Scholar]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Cardenas-Toro, F.P.; Alcázar-Alay, S.C.; Coutinho, J.P.; Godoy, H.T.; Forster-Carneiro, T.; Meireles, M.A.A. Pressurized liquid extraction and low-pressure solvent extraction of carotenoids from pressed palm fiber: Experimental and economical evaluation. Food Bioprod. Process. 2015, 94, 90–100. [Google Scholar] [CrossRef]
- Šafranko, S.; Ćorković, I.; Jerković, I.; Jakovljević, M.; Aladić, K.; Šubarić, D.; Jokić, S. Green extraction techniques for obtaining bioactive compounds from mandarin peel (Citrus unshiu var. Kuno): Phytochemical analysis and process optimization. Foods 2021, 10, 1043. [Google Scholar] [CrossRef]
- Gonçalves Rodrigues, L.G.; Mazzutti, S.; Vitali, L.; Micke, G.A.; Ferreira, S.R.S. Recovery of bioactive phenolic compounds from papaya seeds agroindustrial residue using subcritical water extraction. Biocatal. Agric. Biotechnol. 2019, 22, 101367. [Google Scholar] [CrossRef]
- Gomes, S.V.F.; Portugal, L.A.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P.; David, J.M. Accelerated solvent extraction of phenolic compounds exploiting a Box-Behnken design and quantification of five flavonoids by HPLC-DAD in Passiflora species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Pukalskienė, M.; Pukalskas, A.; Dienaitė, L.; Revinytė, S.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Recovery of bioactive compounds from strawberry (Fragaria × ananassa) pomace by conventional and pressurized liquid extraction and assessment their bioactivity in human cell cultures. Foods 2021, 10, 1780. [Google Scholar] [CrossRef]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorisation of grape stems as a source of phenolic antioxidants by using a sustainable extraction methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef]
- Çam, M.; Hişil, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel Using Pressurized Liquid Extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Elfalleh, W.; Nasri, N.; Sarraï, N.; Guasmi, F.; Triki, T.; Marzougui, N.; Ferchichi, A. Storage protein contents and morphological characters of some Tunisian pomegranate (Punica granatum L.) cultivars. Acta Bot. Gall. 2010, 157, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Thiex, N.J.; Anderson, S.; Gildemeister, B. Crude fat, hexanes extraction, in feed, cereal grain, and forage (Randall/Soxtec/Submersion method): Collaborative study. J. AOAC Int. 2003, 86, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Wagner, H.; Bladt, S. Plant Drug Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996; ISBN 978-3-540-58676-0. [Google Scholar]
- Qu, W.; Breksa, A.P.; Pan, Z.; Ma, H. Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem. 2012, 132, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.G.C.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M.A. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef]
- Jalal, H.; Pal, M.A.; Ahmad, S.R.; Rather, M.; Andrabi, M.; Hamdani, S. Physico-chemical and functional properties of pomegranate peel and seed powder. Pharma Innov. J. 2018, 7, 1127–1131. [Google Scholar]
- Rowayshed, G.; Salama, A.; Abul-Fadl, M.; Akila-Hamza, S.; Emad, M. Nutritional and Chemical Evaluation for Pomegranate (Punica granatum L.) Fruit Peel and Seeds Powders By Products. Middle East J. Appl. Sci. 2013, 3, 169–179. [Google Scholar]
- Kaufman, M.; Wiesman, Z. Pomegranate Oil Analysis with Emphasis on MALDI-TOF/MS Triacylglycerol Fingerprinting. J. Agric. Food Chem. 2007, 55, 10405–10413. [Google Scholar] [CrossRef]
- Galindo, A.; Calín-Sánchez, Á.; Griñán, I.; Rodríguez, P.; Cruz, Z.N.; Girón, I.F.; Corell, M.; Martínez-Font, R.; Moriana, A.; Carbonell-Barrachina, A.A.; et al. Water stress at the end of the pomegranate fruit ripening stage produces earlier harvest and improves fruit quality. Sci. Hortic. (Amsterdam) 2017, 226, 68–74. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Calín-Sánchez, A.; Carbonell-Barrachina, A.; Hernández, F. Changes in quality parameters, proline, antioxidant activity and color of pomegranate (Punica granatum L.) as affected by fruit position within tree, cultivar and ripening stage. Sci. Hortic. 2014, 165, 181–189. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Araújo, P.; Duarte, M.F.; de Freitas, V.; Pintado, M.; Saraiva, J.A. Experimental Design, Modeling, and Optimization of High-Pressure-Assisted Extraction of Bioactive Compounds from Pomegranate Peel. Food Bioprocess Technol. 2017, 10, 886–900. [Google Scholar] [CrossRef]
- Bustamante, A.; Hinojosa, A.; Robert, P.; Escalona, V. Extraction and microencapsulation of bioactive compounds from pomegranate (Punica granatum var. Wonderful) residues. Int. J. Food Sci. Technol. 2017, 52, 1452–1462. [Google Scholar] [CrossRef]
- Kazemi, M.; Karim, R.; Mirhosseini, H.; Abdul Hamid, A. Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: Punicalagin and hydroxybenzoic acids. Food Chem. 2016, 206, 156–166. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Z.; Ma, H.; Atungulu, G.G. Extract of Phenolics From Pomegranate Peels. Open Food Sci. J. 2011, 5, 17–25. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Sumere, B.R.; Mekaru, C.; Martinez, J.; Bezerra, R.M.N.; Rostagno, M.A. Extraction of polyphenols and antioxidants from pomegranate peel using ultrasound: Influence of temperature, frequency and operation mode. Int. J. Food Sci. Technol. 2019, 54, 2792–2801. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, M. Extraction process optimization for bioactive compounds in pomegranate peel. Food Biosci. 2015, 12, 100–106. [Google Scholar] [CrossRef]
- Rajha, H.N.; Abi-Khattar, A.-M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Tamura, K.; Ogo, Y.; Imoto, T. Effect of Pressure on the Solvent Polarity Parameter: ET-Value. Chem. Lett. 1973, 2, 625–628. [Google Scholar] [CrossRef]
- Kronholm, J.; Hartonen, K.; Riekkola, M.-L. Analytical extractions with water at elevated temperatures and pressures. TrAC Trends Anal. Chem. 2007, 26, 396–412. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Tarone, A.G.; Tosi, M.M.; Maróstica Júnior, M.R.; Meireles, M.A.A. Extraction of bioactive compounds from genipap (Genipa americana L.) by pressurized ethanol: Iridoids, phenolic content and antioxidant activity. Food Res. Int. 2017, 102, 595–604. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Cardenas-Toro, F.P.; Osorio-Tobón, J.F.; Barbero, G.F.; Meireles, M.A.d.A. Obtaining anthocyanin-rich extracts from frozen açai (Euterpe oleracea Mart.) pulp using pressurized liquid extraction. Food Sci. Technol. 2017, 37, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Spangenberg, B.; Poole, C.F.; Weins, C. Quantitative Thin-layer Chromatography: A Practical Survey; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9783642107276. [Google Scholar]
- Waksmundzka-Hajnos, M.; Sherma, J.; Kowalska, T. Thin Layer Chromatography in Phytochemistry, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Calín-Sánchez, Á.; Figiel, A.; Hernández, F.; Melgarejo, P.; Lech, K.; Carbonell-Barrachina, Á.A. Chemical Composition, Antioxidant Capacity, and Sensory Quality of Pomegranate (Punica granatum L.) Arils and Rind as Affected by Drying Method. Food Bioprocess Technol. 2013, 6, 1644–1654. [Google Scholar] [CrossRef]
- Qu, W.; Pan, Z.; Ma, H. Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 2010, 99, 16–23. [Google Scholar] [CrossRef]
- Lu, J.; Ding, K.; Yuan, Q. Determination of Punicalagin Isomers in Pomegranate Husk. Chromatographia 2008, 68, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Farías-Campomanes, A.M.; Rostagno, M.A.; Meireles, M.A.A. Production of polyphenol extracts from grape bagasse using supercritical fluids: Yield, extract composition and economic evaluation. J. Supercrit. Fluids 2013, 77, 70–78. [Google Scholar] [CrossRef]
- Legua, P.; Forner-Giner, M.Á.; Nuncio-Jáuregui, N.; Hernández, F. Polyphenolic compounds, anthocyanins and antioxidant activity of nineteen pomegranate fruits: A rich source of bioactive compounds. J. Funct. Foods 2016, 23, 628–636. [Google Scholar] [CrossRef]
- Al-Rawahi, A.S.; Rahman, M.S.; Guizani, N.; Essa, M.M. Chemical Composition, Water Sorption Isotherm, and Phenolic Contents in Fresh and Dried Pomegranate Peels. Dry. Technol. 2013, 31, 257–263. [Google Scholar] [CrossRef]



| Treatments | Temperature (°C) | Pressure (bar) | X0 | Pun α | Pun β | EA |
|---|---|---|---|---|---|---|
| (%) | (mg/100 g dw) | (mg/100 g dw) | (mg/100 g dw) | |||
| 1 | 40 | 20 | 27.41 ± 0.77 c | |||
| 2 | 40 | 40 | 31.01 ± 1.98 b,c | |||
| 3 | 40 | 60 | 30.59 ± 0.38 b,c | |||
| 4 | 40 | 80 | 31.39 ± 0.36 b,c | |||
| 5 | 40 | 100 | 31.12 ± 3.21 b,c | |||
| 6 | 60 | 20 | 30.00 ± 1.96 b,c | 48.22 ± 2.05 a | 146.58 ± 11.20 a | 25.57 ± 0.27 a |
| 7 | 60 | 40 | 37.28 ± 0.41 a,b | |||
| 8 | 60 | 60 | 35.36 ± 1.18 b,c | 40.41 ± 1.56 b | 125.72 ± 3.65 b | 22.74 ± 0.30 b |
| 9 | 60 | 80 | 44.99 ± 1.81 a | |||
| 10 | 60 | 100 | 36.06 ± 1.31 a,b,c |
| Extraction Method | Pomegranate Variety (Origin) | Extraction Solvent | Operation Conditions | Extraction Yield (%) | Target Phenolic Compounds Content (mg/g dw) | Reference |
|---|---|---|---|---|---|---|
| High-Pressure Extraction (HPE) | (Portugal) | 36% ethanol | P = 3820 bar Ө = 30 min | 24.9–31.3% | 3.12 ± 0.4 (α Pun) 3.62 ± 0.4 (β Pun) 691 ± 115 (EA) | [49] |
| Microwave-Assisted Extraction (MAE) | (Rodi Hellas, Greece) | 50% ethanol | S/F = 60/1 MP = 600 W | - | 143.64 (α, β Pun) | [20] |
| Ultrasound-Assisted Extraction (UAE) | Malas (Isfahan, Irán) | 70% ethanol | UIL = 105 W/cm2 Duty cycle = 50% (10 min) OM = pulse | 26.8–41.6% | 128.02-146.61 (α, β Pun) 10.12-22.53 (EA) | [51] |
| (Beirut, Lebanon) | Water | UP = 400 W Ө = 7 min T < 2 °C | - | 0.207 (EA) | [55] | |
| Ultrasound-Assisted Extraction (UAE) | Sishekape-Ferdos | Water | A = 60% Ө = 6.2 min | 13.1% | - | [22] |
| Wonderful (Apulia, Italy) | 70% ethanol | A = 50–80% Ө = 10 min T = 45–70 °C | - | ≈40 µg/mL (EA) | [25] | |
| (Do, Bosnia and Herzegovina, Serbia) | 59% ethanol | Ө = 25 min S/F = 44 T = 80 °C | - | 11.65 ± 0.42 (EA) 2.87 ± 0.11 (GA) 18.05 ± 0.62 (α, β Pun) | [56] | |
| Molar (SP, Brazil) | 70% ethanol | T = 50–60 °C F = 37 KHz OM = normal and pulse | - | 14.8–16.19 (α Pun) 22.45–24.29 (β Pun) 2.13-2.23 (EA) | [53] | |
| Accelerated Solvent Extraction (ASE) | (Turkey) | Water | T = 40 °C P = 103.5 bar | 43.3± 2.7% | 116.6 ± 12.2 (α, β Pun) 1.25 ± 0.2 (EA) | [35] |
| Wonderful (Atacama, Chile) | 77% ethanol | T = 200 °C P = 103.4 bar Ө = 20 min | - | 17 ± 3.6 (α, β Pun) | [36] | |
| Wonderful (California, USA) | 70% ethanol | T = 60 °C P = 100 bar | - | 4.14 ± 0.19 (α Pun) 8.12 ± 0.28 (β Pun) 1.28 ± 0.09 (EA) | [24] | |
| Supercritical Fluid Extraction (SFE) | Wonderful (Elqui valley, Chile) | scCO2: ethanol (80:20) | T = 40–50 °C P = 200–300 bar | 0.2–8.5% | 4–95 (α, β Pun) | [50] |
| Conventional Extraction | Wonderful (California, USA) | Ethanol | T = 40 °C P = 1.01 bar S/F = 15 Ө = 240 min | 17.71% | - | [52] |
| (Himachal Pradesh, India) | 60% ethanol | T = 50 °C P = 1.01 bar S/F = 30 Ө = 45 min | 40–68% | - | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo-Merma, P.R.; Cornejo-Figueroa, M.H.; Crisosto-Fuster, A.d.R.; Strieder, M.M.; Chañi-Paucar, L.O.; Náthia-Neves, G.; Rodríguez-Papuico, H.; Rostagno, M.A.; A. Meireles, M.A.; Alcázar-Alay, S.C. Phenolic Compounds Recovery from Pomegranate (Punica granatum L.) By-Products of Pressurized Liquid Extraction. Foods 2022, 11, 1070. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11081070
Toledo-Merma PR, Cornejo-Figueroa MH, Crisosto-Fuster AdR, Strieder MM, Chañi-Paucar LO, Náthia-Neves G, Rodríguez-Papuico H, Rostagno MA, A. Meireles MA, Alcázar-Alay SC. Phenolic Compounds Recovery from Pomegranate (Punica granatum L.) By-Products of Pressurized Liquid Extraction. Foods. 2022; 11(8):1070. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11081070
Chicago/Turabian StyleToledo-Merma, Pamela R., Marianné H. Cornejo-Figueroa, Anabel d. R. Crisosto-Fuster, Monique M. Strieder, Larry O. Chañi-Paucar, Grazielle Náthia-Neves, Héctor Rodríguez-Papuico, Mauricio A. Rostagno, Maria Angela A. Meireles, and Sylvia C. Alcázar-Alay. 2022. "Phenolic Compounds Recovery from Pomegranate (Punica granatum L.) By-Products of Pressurized Liquid Extraction" Foods 11, no. 8: 1070. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11081070