Physical Properties of Extrudates with Fibrous Structures Made of Faba Bean Protein Ingredients Using High Moisture Extrusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
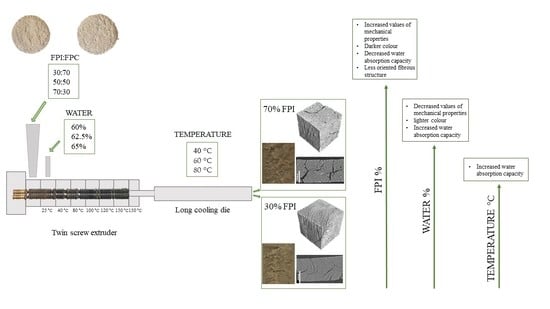
2.2. High Moisture Extrusion
2.3. Mechanical Properties
2.3.1. Texture Profile Analysis
2.3.2. Cutting Strength
2.4. Colour
2.5. Water Absorption Capacity (WAC) and Oil Absorption Capacity (OAC)
2.6. Water Hydration Capacity (WHC)
2.7. Stereomicroscopy
2.8. Microtomography
2.9. Statistical Analyses
3. Results and Discussion
3.1. Regression Models
3.2. Effect of Independent Variables on the Mechanical Properties of Meat Analogues
3.3. The Effect of Independent Variables on Torque and Pressure
3.4. Colour
3.5. Water and Oil Absorption Capacity and Water Holding Capacity of the Meat Analogue
3.6. Microstructure of the Samples


4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Response Variables | Intercept | FPIc (%) | LT (°C) | FWC (%) | R2 | Q2 | F | RMSECV |
|---|---|---|---|---|---|---|---|---|
| Torque | 2.80 | 0.82 | 0.09 | −0.26 | 0.75 | 0.70 | 6.86 ** | 2.99 |
| Pressure | 3.87 | 0.52 | −0.12 | −0.46 | 0.49 | 0.37 | 1.83 | 0.98 |
| Hardness | 2.17 | 0.73 | −0.02 | −0.39 | 0.68 | 0.62 | 4.95 * | 65.32 |
| Springiness | 30.5 | 0.61 | −0.04 | −0.36 | 0.51 | 0.42 | 2.02 | 0.02 |
| Gumminess | 1.51 | 0.80 | 0.04 | −0.33 | 0.74 | 0.70 | 6.87 ** | 46.81 |
| Chewiness | 1.47 | 0.80 | 0.04 | −0.33 | 0.75 | 0.71 | 7.22 ** | 41.91 |
| CS_L | 2.29 | 0.72 | −0.07 | −0.46 | 0.73 | 0.67 | 6.35 ** | 2.15 |
| CS_P | 2.98 | 0.77 | 0.02 | −0.34 | 0.71 | 0.69 | 6.79 ** | 1.23 |
| WAC | 11.65 | −0.60 | 0.19 | 0.61 | 0.77 | 0.67 | 5.92 ** | 6.51 |
| OAC | 6.34 | −0.51 | −0.02 | 0.23 | 0.32 | 0.19 | 0.56 | 2.50 |
| WHC | 31.39 | −0.61 | −0.12 | 0.11 | 0.40 | 0.27 | 1.11 | 0.06 |
| L* | 15.95 | −0.63 | 0.09 | 0.46 | 0.62 | 0.52 | 3.40 * | 1.94 |
| a* | −0.45 | −0.71 | −0.36 | −0.24 | 0.69 | 0.49 | 2.78 | 0.15 |
| b* | 18.49 | −0.18 | 0.13 | 0.30 | 0.14 | −0.15 | 0.00 | 0.54 |
| VIP | 1.49 | 0.29 | 0.83 |
Appendix B
| Sample | FPIc (%) | LT (°C) | FWC (%) | Torque (Nm) | Pressure (bar) | Texture Profile Analysis | Cutting Strength | AI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hardness (N) | Springiness | Gumminess (N) | Chewiness (J) | CS_L (N) | CS_P (N) | |||||||
| 1 | 30 | 60 | 60 | 13.3 ± 0.6 | 4.1 ± 0.3 | 220 ± 20 | 0.9 ± 0.0 | 93 ± 12 | 80 ± 9 | 10 ± 2 | 6.9 ± 0.6 | 0.69 |
| 2 | 30 | 60 | 65 | 10.7 ± 0.3 | 3.4 ± 0.2 | 140 ± 20 | 0.8 ± 0.1 | 53 ± 12 | 45 ± 14 | 5.8 ± 0.4 | 3.8 ± 0.5 | 0.66 |
| 3 | 30 | 40 | 62.5 | 11.7 ± 0.4 | 4.9 ± 0.3 | 223 ± 10 | 0.9 ± 0.0 | 89 ± 7 | 80 ± 5 | 6.4 ± 0.6 | 5.7± 0.1 | 0.89 |
| 4 | 30 | 80 | 62.5 | 12.1 ± 0.4 | 4.1 ± 0.1 | 163 ± 11 | 0.8 ± 0.0 | 65 ± 6 | 54 ± 5 | 6.2 ± 0.3 | 5.7 ± 0.7 | 0.92 |
| 5 | 30 | 60 | 62.5 | 11.3 ± 0.3 | 4.0 ± 0.1 | 168 ± 11 | 0.8 ± 0.1 | 72 ± 6 | 60 ± 8 | 5.4 ± 0.6 | 4.9 ± 0.5 | 0.91 |
| 6 | 70 | 60 | 60 | 24.6 ± 1.2 | 5.7 ± 1.1 | 450 ± 20 | 0.9 ± 0.0 | 300 ± 20 | 270 ± 20 | 18 ± 2 | 11 ± 3 | 0.61 |
| 7 | 70 | 60 | 65 | 22.9 ± 1.0 | 4.4 ± 0.5 | 340 ± 20 | 0.9 ± 0.0 | 220 ± 20 | 196 ± 15 | 11.1 ± 1.5 | 10 ± 2 | 0.85 |
| 8 | 70 | 40 | 62.5 | 24.7 ± 1.2 | 9.0 ± 1.0 | 370 ± 40 | 0.9 ± 0.0 | 250 ± 20 | 220 ± 20 | 13.9 ± 1.5 | 9.1 ± 1.2 | 0.66 |
| 9 | 70 | 80 | 62.5 | 23.6 ± 1.4 | 4.8 ± 0.7 | 440 ± 50 | 0.9 ± 0.0 | 300 ± 40 | 270 ± 30 | 12 ± 3 | 9.8 ± 1.4 | 0.79 |
| 10 | 70 | 60 | 62.5 | 23.0 ± 2.0 | 6.6 ± 1.0 | 310 ± 30 | 0.9 ± 0.0 | 198 ± 12 | 175 ± 11 | 14 ± 2 | 10.2 ± 1.4 | 0.71 |
| 11 | 50 | 40 | 60 | 15.1 ± 0.5 | 5.5 ± 0.6 | 260 ± 30 | 0.9 ± 0.0 | 140 ± 20 | 120 ± 20 | 10.6 ± 0.5 | 7.5 ± 1.3 | 0.71 |
| 12 | 50 | 80 | 60 | 14.6 ± 0.5 | 4.8 ± 0.1 | 260 ± 20 | 0.9 ± 0.0 | 143 ± 10 | 130 ± 8 | 10.8 ± 1.0 | 8 ± 2 | 0.72 |
| 13 | 50 | 60 | 62.5 | 12.6 ± 0.5 | 4.4 ± 0.1 | 158 ± 12 | 0.9 ± 0.0 | 76 ± 8 | 68 ± 7 | 5.4 ± 0.2 | 4.1 ± 0.2 | 0.76 |
| 14 | 50 | 40 | 65 | 10.5 ± 0.4 | 4.7 ± 0.2 | 126 ± 10 | 0.9 ± 0.1 | 55 ± 8 | 48 ± 6 | 6.5 ± 0.8 | 5.2 ± 0.9 | 0.8 |
| 15 | 50 | 60 | 62.5 | 12.1 ± 0.5 | 4.6 ± 0.1 | 160 ± 20 | 0.9 ± 0.0 | 81 ± 9 | 72 ± 8 | 5.1 ± 0.4 | 4.6 ± 0.2 | 0.89 |
| 16 | 50 | 80 | 65 | 10.8 ± 0.4 | 4.0 ± 0.1 | 103 ± 11 | 0.9 ± 0.1 | 39 ± 6 | 33 ± 6 | 5.1 ± 0.7 | 5.4 ± 0.9 | 1.05 |
| 17 | 50 | 60 | 62.5 | 12.0 ± 0.4 | 5.0 ± 0.2 | 155 ± 4.0 | 0.9 ± 0.0 | 73 ± 5 | 67 ± 5 | 5.7 ± 0.6 | 5.2 ± 0.7 | 0.91 |
Appendix C
| Sample | FPIc (%) | LT (°C) | FWC (%) | WAC | OAC | WHC | Colour | ||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||||
| 1 | 30 | 60 | 60 | 134 ± 3 | 16.7 ± 1.5 | 2.3 ± 0.1 | 44.1 ± 1.4 | 0.3 ± 0.2 | 8.9 ± 0.4 |
| 2 | 30 | 60 | 65 | 155 ± 12 | 19.0 ± 2.0 | 2.4 ± 0.1 | 49.4 ± 1.3 | 0.07 ± 0.10 | 10.1 ± 0.7 |
| 3 | 30 | 40 | 62.5 | 140 ± 4 | 25.0 ± 5.0 | 2.4 ± 0.1 | 48.2 ± 1.0 | 0.25 ± 0.15 | 10.4 ± 0.5 |
| 4 | 30 | 80 | 62.5 | 150 ± 3 | 18.0 ± 2.0 | 2.4 ± 0.1 | 47.2 ± 1.4 | −0.06 ± 0.13 | 9.0 ± 1.0 |
| 5 | 30 | 60 | 62.5 | 151 ± 1 | 20.8 ± 1.3 | 2.4 ± 0.0 | 49.9 ± 1.2 | 0.01 ± 0.18 | 9.85 ± 0.9 |
| 6 | 70 | 60 | 60 | 126 ± 2 | 16.1 ± 0.5 | 2.2 ± 0.0 | 39.7 ± 0.7 | −0.2 ± 0.2 | 8.8 ± 0.4 |
| 7 | 70 | 60 | 65 | 134 ± 2 | 18.0 ± 2.0 | 2.3 ± 0.1 | 46.3 ± 0.8 | −0.46 ± 0.06 | 9.3 ± 0.1 |
| 8 | 70 | 40 | 62.5 | 121 ± 2 | 15.0 ± 2.0 | 2.3 ± 0.0 | 44.1 ± 1.0 | −0.06 ± 0.13 | 10.2 ± 0.4 |
| 9 | 70 | 80 | 62.5 | 127 ± 3 | 15.0 ± 2.0 | 2.3 ± 0.0 | 43.9 ± 0.7 | −0.31 ± 0.14 | 9.9 ± 0.4 |
| 10 | 70 | 60 | 62.5 | 129 ± 2 | 17.7 ± 0.7 | 2.2 ± 0.0 | 43.7 ± 1.2 | −0.2 ± 0.2 | 9.9 ± 0.4 |
| 11 | 50 | 40 | 60 | 110 ± 3 | 13.0 ± 4.0 | 2.4 ± 0.1 | 46.0 ± 1.5 | −0.07 ± 0.09 | 9.2 ± 0.3 |
| 12 | 50 | 80 | 60 | 123 ± 10 | 16.0 ± 4.0 | 2.3 ± 0.0 | 44.7 ± 1.0 | −0.19 ± 0.08 | 9.6 ± 0.4 |
| 13 | 50 | 60 | 62.5 | 133 ± 7 | 21.0 ± 6.0 | 2.2 ± 0.0 | 49.9 ± 0.6 | −0.22 ± 0.09 | 9.8 ± 0.6 |
| 14 | 50 | 40 | 65 | 137 ± 6 | 15.2 ± 0.7 | 2.4 ± 0.0 | 50.0 ± 1.0 | 0.14 ± 0.08 | 10.7 ± 0.3 |
| 15 | 50 | 60 | 62.5 | 144 ± 4 | 19.0 ± 3.0 | 2.4 ± 0.0 | 48.0 ± 1.4 | −0.25 ± 0.04 | 9.94 ± 0.3 |
| 16 | 50 | 80 | 65 | 142 ± 5 | 18.0 ± 3.0 | 2.3 ± 0.1 | 48.4 ± 0.9 | −0.25 ± 0.06 | 10.0 ± 0.2 |
| 17 | 50 | 60 | 62.5 | 136 ± 5 | 20.0 ± 4.0 | 2.5 ± 0.0 | 49.4 ± 1.3 | −0.23 ± 0.07 | 9.8 ± 0.3 |
References
- Curtain, F.; Grafenauer, S. Plant-Based Meat Substitutes in the Flexitarian Age: An Audit of Products on Supermarket Shelves. Nutrients 2019, 11, 2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sha, L.; Xiong, Y.L. Plant Protein-Based Alternatives of Reconstructed Meat: Science, Technology, and Challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Bashi, Z.; McCullough, R.; Ong, L.; Ramirez, M. Alternative Proteins: The Race for Market Share Is On; McKinsey & Company: Denver, CO, USA, 2019; pp. 1–11. [Google Scholar]
- Lizarazo, C.I.; Lampi, A.; Liu, J.; Sontag-Strohm, T.; Piironen, V.; Stoddard, F.L. Nutritive Quality and Protein Production from Grain Legumes in a Boreal Climate. J. Sci. Food Agric. 2015, 95, 2053–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoddard, F.L.; Stoddard, F.L.; Hovinen, S.; Kontturi, M.; Lindström, K.; Nykänen, A. Legumes in Finnish Agriculture: History, Present Status and Future Prospects. Agric. Food Sci. 2009, 18, 191–205. [Google Scholar] [CrossRef] [Green Version]
- Warsame, A.O.; O’Sullivan, D.M.; Tosi, P. Seed Storage Proteins of Faba Bean (Vicia faba L.): Current Status and Prospects for Genetic Improvement. J. Agric. Food Chem. 2018, 66, 12617–12626. [Google Scholar] [CrossRef]
- Sosulski, F.W.; McCurdy, A.R. Functionality of Flours, Protein Fractions and Isolates from Field Peas and Faba Bean. J. Food Sci. 1987, 52, 1010–1014. [Google Scholar] [CrossRef]
- Cai, R.; McCurdy, A.; Baik, B. Textural Property of 6 Legume Curds in Relation to Their Protein Constituents. J. Food Sci. 2002, 67, 1725–1730. [Google Scholar] [CrossRef]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Kim, T.; Riaz, M.N.; Awika, J.; Teferra, T.F. The Effect of Cooling and Rehydration Methods in High Moisture Meat Analogs with Pulse Proteins-Peas, Lentils, and Faba Beans. J. Food Sci. 2021, 86, 1322–1334. [Google Scholar] [CrossRef]
- Jiang, Z.-Q.; Wang, J.; Stoddard, F.; Salovaara, H.; Sontag-Strohm, T. Preparation and Characterization of Emulsion Gels from Whole Faba Bean Flour. Foods 2020, 9, 755. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Knutsen, S.H.; Malizia, G.; Dessev, T.; Geny, A.; Zobel, H.; Myhrer, K.; Varela, P.; Sahlstrøm, S. Meat Analogues from a Faba Bean Concentrate Can Be Generated by High Moisture Extrusion. Future Foods 2021, 3, 100014. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-Moisture Meat Analogues Produced from Yellow Pea and Faba Bean Protein Isolates/Concentrate: Effect of Raw Material Composition and Extrusion Parameters on Texture Properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef]
- Kim, T.; Miller, R.; Laird, H.; Riaz, M.N. Beef Flavor Vegetable Hamburger Patties with High Moisture Meat Analogs (HMMA) with Pulse Proteins- Peas, Lentils, and Faba Beans. J. Food Sci. 2021, 9, 4048–4056. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring Processes for Meat Analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Guyony, V.; Fayolle, F.; Jury, V. High Moisture Extrusion of Vegetable Proteins for Making Fibrous Meat Analogs: A Review. Food Rev. Int. 2022, 1–26. [Google Scholar] [CrossRef]
- Vatansever, S.; Tulbek, M.C.; Riaz, M.N. Low-and High-Moisture Extrusion of Pulse Proteins as Plant-Based Meat Ingredients: A Review. Cereal Foods World 2020, 65, 12–14. [Google Scholar]
- Schutyser, M.A.I.; Pelgrom, P.J.M.; Van der Goot, A.J.; Boom, R.M. Dry Fractionation for Sustainable Production of Functional Legume Protein Concentrates. Trends Food Sci. Technol. 2015, 45, 327–335. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying Properties of Chickpea, Faba Bean, Lentil and Pea Proteins Produced by Isoelectric Precipitation and Salt Extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Martinez, M.; Stone, A.K.; Yovchev, A.G.; Peter, R.; Vandenberg, A.; Nickerson, M.T. Effect of Genotype and Environment on the Surface Characteristics and Functionality of Air-Classified Faba Bean Protein Concentrates. Eur. Food Res. Technol. 2016, 242, 1903–1911. [Google Scholar] [CrossRef]
- Lampi, A.M.; Damerau, A.; Li, J.; Moisio, T.; Partanen, R.; Forssell, P.; Piironen, V. Changes in Lipids and Volatile Compounds of Oat Flours and Extrudates during Processing and Storage. J. Cereal Sci. 2015, 62, 102–109. [Google Scholar] [CrossRef]
- Liu, M.; Lampi, A.M.; Ertbjerg, P. Unsaturated Fat Fraction from Lard Increases the Oxidative Stability of Minced Pork. Meat Sci. 2018, 143, 87–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC Association of Official Analytical Chemists, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005.
- Dansby, M.Y.; Bovell-Benjamin, A.C. Physical Properties and Sixth Graders’ Acceptance of an Extruded Ready-to-Eat Sweetpotato Breakfast Cereal. J. Food Sci. 2003, 68, 2607–2612. [Google Scholar] [CrossRef] [PubMed]
- Ramos Diaz, J.M.; Kantanen, K.; Edelmann, J.M.; Suhonen, H.; Sontag-Strohm, T.; Jouppila, K.; Piironen, V. Fibrous Meat Analogues Containing Oat Fiber Concentrate and Pea Protein Isolate: Mechanical and Physicochemical Characterization. Innov. Food Sci. Emerg. Technol. 2022, 77, 102954. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.E.; Hsieh, F. Extrusion Process Parameters, Sensory Characteristics, and Structural Properties of a High Moisture Soy Protein Meat Analog. J. Food Sci. 2002, 67, 1066–1072. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Buades, A.; Bartomeu, C.; Morel, J.-M. Non-Local Means Denoising. Image Process. Line 2011, 1, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Darbon, J.; Cunha, A.; Chan, T.F.; Osher, S.; Jensen, G.J. Fast Nonlocal Filtering Applied to Electron Cryomicroscopy. In Proceedings of the 2008 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Paris, France, 14–17 May 2008; pp. 1331–1334. [Google Scholar]
- Dougherty, R.; Kunzelmann, K.-H. Computing Local Thickness of 3D Structures with ImageJ. Microsc. Microanal. 2007, 13, 1678–1679. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Zhu, S.; Wang, Q. Texturisation Behaviour of Peanut–Soy Bean/Wheat Protein Mixtures during High Moisture Extrusion Cooking. Int. J. Food Sci. Technol. 2018, 53, 2535–2541. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.E.; Hsieh, F. Texture and Chemical Characteristics of Soy Protein Meat Analog Extruded at High Moisture. J. Food Sci. 2000, 65, 264–269. [Google Scholar] [CrossRef]
- Palanisamy, M.; Franke, K.; Berger, R.G.; Heinz, V.; Töpfl, S. High Moisture Extrusion of Lupin Protein: Influence of Extrusion Parameters on Extruder Responses and Product Properties. J. Sci. Food Agric. 2019, 99, 2175–2185. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B.; Ojokoh, A.O. System Parameters and Product Properties Response of Soybean Protein Extruded at Wide Moisture Range. J. Food Eng. 2010, 96, 208–213. [Google Scholar] [CrossRef]
- Wittek, P.; Karbstein, H.P.; Emin, M.A. Blending Proteins in High Moisture Extrusion to Design Meat Analogues: Rheological Properties, Morphology Development and Product Properties. Foods 2021, 10, 1509. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High Moisture Extrusion Cooking of Pea Protein Isolates: Raw Material Characteristics, Extruder Responses, and Texture Properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Meng, S.; Wang, Q. Rheological Properties of Pea Protein Isolate-Amylose/Amylopectin Mixtures and the Application in the High-Moisture Extruded Meat Substitutes. Food Hydrocoll. 2021, 117, 106732. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Bühler, J.M.; Karbstein, H.P.; Emin, M.A. High Moisture Extrusion of Soy Protein Concentrate: Influence of Thermomechanical Treatment on Protein-Protein Interactions and Rheological Properties. J. Food Eng. 2019, 251, 11–18. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Faisal, S.; Wang, Q. A New Insight into the High-Moisture Extrusion Process of Peanut Protein: From the Aspect of the Orders and Amount of Energy Input. J. Food Eng. 2020, 264, 109668. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, B.; Wei, Y. Effects of the Specific Mechanical Energy on the Physicochemical Properties of Texturized Soy Protein during High-Moisture Extrusion Cooking. J. Food Eng. 2014, 121, 32–38. [Google Scholar] [CrossRef]
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional Composition, Anti-Nutritional Factors, Pretreatments-Cum-Processing Impact and Food Formulation Potential of Faba Bean (Vicia faba L.): A Comprehensive Review. Compr. Rev. 2021, 138, 110796. [Google Scholar] [CrossRef]
- Samard, S.; Ryu, G.-H. A Comparison of Physicochemical Characteristics, Texture, and Structure of Meat Analogue and Meats. J. Sci. Food Agric. 2019, 99, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gu, B.-Y.; Ryu, G.-H. Optimization of Extrusion Variables for Improving the Qualities of Textured Vegetable Protein with Green Tea Using Response Surface Methodology. Food Eng. Prog. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Kaleda, A.; Talvistu, K.; Tamm, M.; Viirma, M.; Rosend, J.; Tanilas, K.; Kriisa, M.; Part, N.; Tammik, M.-L. Impact of Fermentation and Phytase Treatment of Pea-Oat Protein Blend on Physicochemical, Sensory, and Nutritional Properties of Extruded Meat Analogs. Foods 2020, 9, 1059. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Liu, L.; Zhang, Y.; He, N.; Wang, Q. High-Moisture Extrusion Process of Transglutaminase-Modified Peanut Protein: Effect of Transglutaminase on the Mechanics of the Process Forming a Fibrous Structure. Food Hydrocoll. 2021, 112, 106346. [Google Scholar] [CrossRef]
- Geerts, M.E.J.; Dekkers, B.L.; van der Padt, A.; van der Goot, A.J. Aqueous Fractionation Processes of Soy Protein for Fibrous Structure Formation. Innov. Food Sci. Emerg. Technol. 2018, 45, 313–319. [Google Scholar] [CrossRef]






| Content g/100 g DM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw Material | Protein | Dietary Fibre | Fat | Starch | WSI | WAI | Particle Size | |||
| Insoluble | Soluble | Total | D [2,3] | D [3,4] | ||||||
| FPI | 80.1 | 9.7 | 1.0 | 10.7 | 4.8 | 0.3 | 11.6 ± 1.7 | 481 ± 7 | 32.7 ± 0.7 | 50.8 ± 0.3 |
| FPC | 55.2 | 17.6 | 2.3 | 19.9 | 4.9 | 7.3 | 56.3 ± 0.1 | 162 ± 1 | 6.8 ± 0.1 | 11.4 ± 0.1 |
| Mixtures FPI:FPC | ||||||||||
| 70:30 | 72.6 * | 12.1 * | 1.4 * | 13.5 * | 4.8 * | 2.4 * | 24.6 ± 0.8 | 290 ± 14 | 15.6 ± 0.6 | 39.6 ± 0.2 |
| 50:50 | 67.6 * | 13.7 * | 1.7 * | 15.4 * | 4.8 * | 3.8 * | 31.2 ± 0.9 | 271 ± 30 | 10.2 ± 1.1 | 27.9 ± 4.5 |
| 30:70 | 62.7 * | 15.3 * | 1.9 * | 17.2 * | 4.9 * | 5.2 * | 41.9 ± 0.5 | 216 ± 1 | 9.3 ± 0.1 | 25.8 ± 0.4 |
| Experiment Number | FPIc (% of Solids) | LT (°C) | FWC (%) |
|---|---|---|---|
| 1 | 30 | 60 | 60 |
| 2 | 30 | 60 | 65 |
| 3 | 30 | 40 | 62.5 |
| 4 | 30 | 80 | 62.5 |
| 5 | 30 | 60 | 62.5 |
| 6 | 70 | 60 | 60 |
| 7 | 70 | 60 | 65 |
| 8 | 70 | 40 | 62.5 |
| 9 | 70 | 80 | 62.5 |
| 10 | 70 | 60 | 62.5 |
| 11 | 50 | 40 | 60 |
| 12 | 50 | 80 | 60 |
| 13 | 50 | 60 | 62.5 |
| 14 | 50 | 40 | 65 |
| 15 | 50 | 60 | 62.5 |
| 16 | 50 | 80 | 65 |
| 17 | 50 | 60 | 62.5 |
| Torque | Pressure | Hardness b | Springiness a | Gumminess b | Chewiness b | CS_L | CS_P | WAC | OAC a | WHC a | L* | a* b | b* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constant | 12.2 *** | 4.73 *** | 149.8 *** | 0.90 *** | 70.7 *** | 63.2 *** | 5.61 *** | 4.81 *** | 137.3 *** | 19.6 *** | 2.36 *** | 49.2 *** | −0.23 * | 9.9 *** |
| FPIc | 6.00 *** | 0.99 ** | 100.5 *** | 0.02 ** | 89.0 *** | 81.5 *** | 3.55 *** | 2.22 *** | −9.12 *** | −1.76 * | −0.06 | −2.11 *** | −0.18 ** | −0.03 |
| FWC | −1.6 *** | −0.47 | −60.2 *** | −0.01 | −39.0 ** | −35.9 ** | −2.56 *** | −1.14 *** | 9.35 *** | 1.05 | 0.02 | 2.46 *** | −0.06 | 0.44 ** |
| LT | −0.11 | −0.8 ** | −1.54 | 0.00 | 0.81 | 1.5 | −0.37 | 0.14 | 4.11 * | −0.32 | −0.02 | −0.51 | −0.13 ** | −0.25 |
| FPIc * FPIc | 5.41 *** | 0.38 | 115.6 ** | −0.02 | 81.3 *** | 71.7 *** | 3.69 *** | 2.22 *** | 2.96 | 0.02 | −0.04 | −2.74 ** | 0.12 | −0.23 |
| FWC * FWC | 0.24 | −0.66 | 16.2 | 0.00 | 8.87 | 9.36 | 1.95 *** | 0.86 * | −3.15 | −2.32 | −0.01 | −1.47 * | 0.07 | −0.35 |
| LT * LT | 0.41 | 0.64 | 26.8 | −0.01 | 19.27 | 15.7 | 0.55 | 0.69 * | −5.95 * | −1.59 | 0.00 | −0.56 | 0.07 | 0.26 |
| FPIc * FWC | 0.22 | −0.11 | −7.91 | 0.00 | −10.33 | −10.8 | −0.52 | 0.46 | −3.41 | −0.21 | 0.02 | 0.32 | 0.00 | −0.19 |
| FPIc * LT | −0.37 | −0.83 * | 31.3 | 0.02 * | 17.41 | 18.4 | −0.33 | 0.15 | −1.03 | 1.72 | 0.01 | 0.20 | 0.02 | 0.28 |
| FWC * LT | 0.19 | −0.02 | −7.42 | −0.01 | −4.75 | −4.9 | −0.41 | −0.04 | −2.06 | 0.17 | 0.02 | −0.07 | −0.07 | −0.29 |
| R2 | 0.99 | 0.90 | 0.97 | 0.82 | 0.97 | 0.97 | 0.99 | 0.97 | 0.94 | 0.70 | 0.51 | 0.95 | 0.84 | 0.85 |
| Q2 | 0.95 | −0.08 | 0.83 | −0.15 | 0.83 | 0.84 | 0.94 | 0.85 | 0.57 | −2.57 | −1.75 | 0.66 | −0.81 | −0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantanen, K.; Oksanen, A.; Edelmann, M.; Suhonen, H.; Sontag-Strohm, T.; Piironen, V.; Ramos Diaz, J.M.; Jouppila, K. Physical Properties of Extrudates with Fibrous Structures Made of Faba Bean Protein Ingredients Using High Moisture Extrusion. Foods 2022, 11, 1280. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11091280
Kantanen K, Oksanen A, Edelmann M, Suhonen H, Sontag-Strohm T, Piironen V, Ramos Diaz JM, Jouppila K. Physical Properties of Extrudates with Fibrous Structures Made of Faba Bean Protein Ingredients Using High Moisture Extrusion. Foods. 2022; 11(9):1280. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11091280
Chicago/Turabian StyleKantanen, Katja, Anni Oksanen, Minnamari Edelmann, Heikki Suhonen, Tuula Sontag-Strohm, Vieno Piironen, Jose Martin Ramos Diaz, and Kirsi Jouppila. 2022. "Physical Properties of Extrudates with Fibrous Structures Made of Faba Bean Protein Ingredients Using High Moisture Extrusion" Foods 11, no. 9: 1280. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11091280