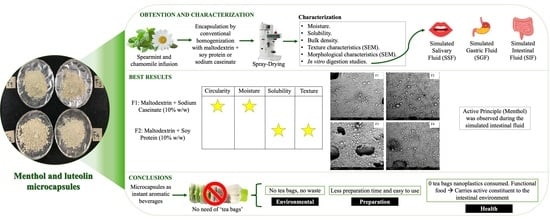
Encapsulation of Menthol and Luteolin Using Hydrocolloids as Wall Material to Formulate Instant Aromatic Beverages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Design
2.2.2. Obtaining the Instant Powders
2.2.3. Instant Powder Characterization
Morphology and Surface Properties
Moisture
Bulk Density
Solubility
Controlled Release of the Powder Contents
- Simulated Salivary Fluid (SSF)
- Simulated Gastric Fluid (SGF)
- Simulated Intestinal Fluid (SIF)
Concentration of Menthol Released
3. Results and Discussion
3.1. Morphology
3.2. Surface Properties
3.3. Moisture
3.4. Bulk Density
3.5. Solubility
3.6. Controlled Release of the Compounds of Interest (Menthol)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fretes, F. Plantas Medicinales y Aromáticas–Una Alternativa de Producción Comercial. USAID. Available online: https://www.usaid.gov/sites/default/files/documents/1862/plantas_medicinales.pdf (accessed on 16 September 2021).
- Khan, M.I.; Maqsood, M.; Saeed, R.A.; Alam, A.; Sahar, A.; Kieliszek, M.; Miecznikowski, A.; Muzammil, H.S.; Aadil, R.M. Phytochemistry, Food Application, and Therapeutic Potential of the Medicinal Plant (Withania coagulans): A Review. Molecules 2021, 26, 6881. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Rabail, R.; Rakha, A.; Bryla, M.; Roszko, M.; Aadil, R.M.; Kieliszek, M. Delving the Role of Caralluma fimbriata: An Edible Wild Plant to Mitigate the Biomarkers of Metabolic Syndrome. Oxidative Med. Cell. Longev. 2022, 2022, 5720372. [Google Scholar] [CrossRef] [PubMed]
- Berdonces, J.L. Historia de la fitoterapia. Nat. Medicatrix 2003, 21, 142–152. [Google Scholar]
- ICA. Plantas Aromáticas y Medicinales, Enfermedades de Importancia y Sus Usos Terapéuticos–Medidas Para la Temporada Invernal. Produmedios. Available online: https://www.ica.gov.co/getattachment/2c392587-f422-4ff5-a86f-d80352f0aa11/Plantas-aromaticas-y-medicinales-Enfermedades-de.aspx (accessed on 16 September 2021).
- Grande Tovar, C.D.; Ospina, J.D. Cadena de valor de Plantas Aromáticas, Medicinales y Condimentarias, 1st ed.; Universidad de San Buenaventura: Cali, Colombia, 2015. [Google Scholar]
- Nayak, P.; Kumar, T.; Gupta, A.K.; Joshi, N.U. Peppermint a medicinal herb and treasure of health: Review. J. Pharmacogn. Phytochem. 2020, 9, 1519–1528. [Google Scholar]
- Srivastava, J.K.; Gupta, S. Chamomile: A Herbal Agent for Treatment of Diseases of the Elderly. In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults, 1st ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; p. 172. [Google Scholar] [CrossRef]
- Cáseres, A. Plantas de Uso Medicinal en Guatemala, 1st ed.; Universidad de San Carlos de Guatemala: Guatemala City, Guatemala, 1999; pp. 265–267. [Google Scholar]
- Kapp, K.; Hakala, E.; Orav, A.; Pohjala, L.; Vuorela, P.; Püssa, T.; Real, A. Commercial peppermint (Mentha x piperita L.) teas: Antichlamydial effect and polyphenolic composition. Food Res. Int. 2013, 53, 758–766. [Google Scholar] [CrossRef]
- Ministerio de la Protección Social. Vademécum Colombiano de Plantas Medicinales; Arte y Sistemas Integrados Ltda: Bogotá, Colombia, 2008.
- Sagduyu, K. Peppermint oil for irritable bowel syndrome. Psychosomatics 2002, 43, 508–509. [Google Scholar] [CrossRef]
- De la Paz, N.J.; Corral, S.A.; Martínez, R.C.; Martínez, M.S. Efecto antidiarreico de la tintura al 20% de Mentha piperita L. en ratas. Rev. Cuba. De Farmacol. 2004, 38, 11–2007. [Google Scholar]
- Parra, A.Q.; de Jesús Castro, M.; Ricaurte, C.E. Determinación de metales en las estructuras del diente de león (Taraxacum officinalis weber) hierbabuena (Mentha piperita) y manzanilla (Matricaria chamomilla). Bistua Rev. De La Fac. De Cienc. Básicas 2005, 3, 38–44. [Google Scholar]
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef]
- Shikov, A.; Pozharitskaya, O.; Makarfov, V.; Kvetnava, A. Antibacterial activity of Chamomilla recutita oil extract against Helicobacter pylory. Phytother. Res. 2007, 22, 252–253. [Google Scholar] [CrossRef]
- Moron, F.; Furones, J.; Pinedo, Z. Actividad espasmódica del extracto fluido de Matricaria recutita (manzanilla) en órganos aislados. Rev. Cuba. De Plantas Med. 1996, 1, 19–24. [Google Scholar]
- Thorne Research Inc. Matricaria chamomilla (German chamomile). Altern. Med. Rev. 2008, 13, 58–62. [Google Scholar]
- Icahn School of Medicine at Mount Sinai. Roman Chamomile. Mount Sinai Health System. Available online: https://www.mountsinai.org/health-library/herb/roman-chamomile#:~:text=Roman%20chamomile%20is%20considered%20generally,also%20be%20allergic%20to%20chamomile (accessed on 22 September 2021).
- Committee on Herbal Medicinal Products (HMPC). European Union Herbal Monograph on Mentha x Piperita L., Folium. European Medicines Agency. 2017. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-mentha-x-piperita-l-folium-revision-1_en.pdf (accessed on 22 September 2021).
- Euromonitor International. Tea in Colombia. Passport. Available online: https://www.euromonitor.com/tea-in-colombia/report (accessed on 28 September 2021).
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Lee, N.; Kim, S.; Lee, J. Valorization of waste tea bags via CO2-assisted pyrolysis. J. CO2 Util. 2020, 44, 101414. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Amaya, J.S.; Segura, S.; Salcedo, F.; Arenas, I.; Rincón, C.; Hernandez, M. Formulation of a responsive in vitro digestion wall material, sensory and market analyses for chia seed oil capsules. J. Food Eng. 2021, 296, 110460. [Google Scholar] [CrossRef]
- Soottitantawat, A.; Takayama, K.; Okamura, K.; Muranaka, D.; Yoshii, H.; Furuta, T.; Ohkawara, M.; Linko, P. Microencapsulation of I-menthol by spray drying and its release characteristics. Innov. Food Sci. Emerg. Technol. 2005, 6, 163–170. [Google Scholar] [CrossRef]
- Eratte, D.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikan, B. Survival, oxidative stability, and surface characteristics of spray dried co-microcapsules containing omega-3 fatty acids and probiotic bacteria. Dry. Technol. 2016, 34, 1926–1935. [Google Scholar] [CrossRef]
- Nielsen, S.S. Determination of Moisture Content, 2nd ed.; Food Analysis Laboratory Manual. Food Science Texts Series; Springer: Boston, MA, USA, 2010; pp. 17–27. [Google Scholar] [CrossRef]
- Abdullah, E.C.; Geldart, D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999, 102, 151–165. [Google Scholar] [CrossRef]
- Artamonov, V.V.; Bykov, A.O.; Bykov, P.O.; Artamonov, V.P. Measurement of the tap density of metal powders. Powder Metall. Met. Ceram. 2013, 52, 237–239. [Google Scholar] [CrossRef]
- Serna-Cock, L.; Torres-León, C.; Ayala-Aponte, A. Evaluación de Polvos Alimentarios obtenidos de Cáscaras de Mango (Mangifera indica) como fuente de Ingredientes Funcionales. Inf. Technol. 2015, 26, 41–50. [Google Scholar] [CrossRef]
- Largo-Ávila, E.; Cortés, M.; Ciro, H.J. Influence of Maltodextrin and Spray Drying Process Conditions on Sugarcane Juice Powder Quality. Rev. Fac. Nac. De Agron. Medellin 2015, 68, 7509–7520. [Google Scholar] [CrossRef]
- Anastasia-Sandu, A.; Bîrzu, S.; Ditu, I.; Bulgariu, L. Direct determination of menthol using a simple spectrophotometric method. Bul. Inst. Politeh. Iaşi 2013, 59, 72–80. [Google Scholar]
- Kushwah, H.; Hans, T.; Chauhan, M.; Mittal, G.; Sandal, N. Development and Validation of the Spectrophotometric Method for the Determination of Menthol. J. Appl. Spectrosc. 2020, 87, 563–567. [Google Scholar] [CrossRef]
- Dangwal, S.K. Colorimetric methods of determination of menthol in air. Ind. Health 1980, 18, 187–193. [Google Scholar] [CrossRef]
- Naji, A.M.; Basyigit, B.; Alasalvar, H.; Salum, P.; Berktas, S.; Erbay, Z.; Cam, M. Instant soluble roselle (Hibiscus sabdariffa L.). J. Food Meas. Charact. 2022, 17, 108–120. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Peighambardoust, S.H.; Sarabandi, K.; Jafari, S.M. Spray drying ecapsulation of bioactive compounds within protein-based carriers; different options and applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Jakstas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Bernatoniene, J. Microencapsulation of Elsholtzia ciliata Herb Ethanolic Extract by Spray-Drying: Impact of Resistant-Maltodextrin Complemented with Sodium Caseinate, Skim Milk, and Beta-Cyclodextrin on the Quality of Spray-Dried Powders. Molecules 2019, 24, 1461. [Google Scholar] [CrossRef]
- Samborska, K.; Langa, E.; Kamińska-Dwórznicka, A.; Witrowa-Rajchert, D. The influence of sodium caseinate on the physical properties of spray-dried honey. Int. J. Food Sci. Technol. 2015, 50, 256–262. [Google Scholar] [CrossRef]
- Cuq, B.; Mandato, S.; Jeantet, R.; Saleh, K.; Ruiz, T. Agglomeration/granulation in food powder production. In Handbook of Food Powders, 1st ed.; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Cambridge, UK, 2013; pp. 150–177. [Google Scholar]
- Augustin, M.; Margetts, C. POWDERED MILK|Milk Powders in the Marketplace. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Trugo, L.C., Finglas, P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 4694–4702. [Google Scholar]
- Syll, O.; Khalloufi, S.; Schuck, P. Dispersibility and morphology of spray-dried soy powders depending on the spraying system. Dairy Sci Technol. 2013, 93, 431–442. [Google Scholar] [CrossRef]
- Fang, Y.; Selomulya, C.; Chen, X.D. On Measurement of Food Powder Reconstitution Properties. Dry. Technol. 2007, 26, 3–14. [Google Scholar] [CrossRef]
- Hogan, S.A.; McNamee, B.F.; O’Riordan, E.D.; O’Sullivan, M. Microencapsulating Properties of Sodium Caseinate. J. Agric. Food Chem. 2001, 49, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Selomulya, C.; Fang, Y. Food powder rehydration. In Handbook of Food Powders, 1st ed; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Cambridge, UK, 2013; pp. 379–408. [Google Scholar]
- Shahidi, F.; Han, X. Encapsulation of food ingredients. Crit. Rev. Food Sci. Nutr. 1993, 33, 501–547. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kadam, D.; Chadha, S.; Wilson, R.; Gupta, R. Influence of particle size on physical and sensory attributes of mango pulp powder. Int. Agrophys. 2013, 27, 323–328. [Google Scholar] [CrossRef]
- Both, E.; Boom, R.; Schutyser, M. Particle morphology and powder properties during spray drying of maltodextrin and whey protein mixtures. Powder Technol. 2020, 363, 519–524. [Google Scholar] [CrossRef]
- Hernández-Carrión, M.; Hernando, I.; Sotelo-Díaz, I.; Quintanilla-Carvajal, M.X.; Quiles, A. Use of image analysis to evaluate the effect of high hydrostatic pressure and pasteurization as preservation treatments on the microstructure of red sweet pepper. Innov. Food Sci. Emerg. Technol. 2015, 27, 69–78. [Google Scholar] [CrossRef]
- Barrera, G.; Calderón-Domínguez, G.; Chanona-Pérez, J.; Gutiérrez-López, G.F.; León, A.E.; Ribotta, P.D. Evaluation of the mechanical damage on wheat starch granules by SEM, ESEM, AFM and textur image analysis. Carbohydr. Polym. 2013, 98, 1449–1457. [Google Scholar] [CrossRef]
- Yang, X.; Beyenal, H.; Harkin, G.; Lewandowski, Z. Quantifying biofilm structure using image analysis. J. Microbiol. Methods 2000, 39, 109–119. [Google Scholar] [CrossRef]
- Laddi, A.; Sharma, S.; Kumar, A.; Kapur, A. Classification of tea grains based upon image texture feature analysis under different illumination conditions. J. Food Eng. 2013, 115, 226–231. [Google Scholar] [CrossRef]
- Bianco, H.; Capote, T.; Garmendia, C. Determinación de humedad en harina precocida de maíz blanco utilizando un horno de microondas doméstico. INHRR 2014, 45. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0798-04772014000200004 (accessed on 10 October 2021).
- Gómez, J. Caracterización Granulométrica de un Producto Comercial en Polvo (Suplemento Dietario) y Evaluación de la Capacidad de Dispersión en Agua. Undergraduate Thesis, Degree-Granting Universidad ICESI, Cali, Colombia, 2016. [Google Scholar]
- Martínez Iturralde, A. Efecto de la Maltodextrina y la Temperatura Sobre Kiwi Deshidratado por Atomización. Undergraduate Thesis, Degree-Granting Universidad Pública de Navarra, Navarra, Spain, 2015. [Google Scholar]
- Iwuozor, K.O.; Adeniyi, A.G.; Emenike, E.C.; Adepoju, M.I.; Ahmed, M.O. Sugarcane Juice Powder Produced from Spray Drying Technology: A Review of Properties and Operating Parameters. Sugar Tech 2023, 25, 497–507. [Google Scholar] [CrossRef]
- Brady, N.C. Soil Density. In The Nature and Properties of Soils, 9th ed.; Macmillan Publishers: Hampshire, UK, 1984. [Google Scholar]
- Calín-Sánchez, A.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Bai, X.; Li, C.; Yu, L.; Jiang, Y.; Wang, M.; Lang, S.; Liu, D. Development and characterization of soybean oil microcapsules employing kafirin and sodium caseinate as wall materials. LWT 2019, 111, 235–241. [Google Scholar] [CrossRef]
- Jafari, S.M.; Ghalegi Ghalenoei, M.; Dehnad, D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Trimedona, N.; Rahzarni; Muchrida, Y.; Amurita-Zebua, E.; Satria-Utama, R. Physicochemical properties of instant beverage powders from red dragon frui peel extracts with maltodextrin and cocoa powder as fillers. IOP Conf. Ser. Earth Environ. Sci. 2022, 1097, 012037. [Google Scholar] [CrossRef]
- Cam, M.; Basyigit, B.; Alasalvar, H.; Yilmaztekin, M.; Ahhmed, A.; Sagdic, O.; Konca, Y.; Telci, I. Bioactive properties of powdered peppermint and spearmint extracts: Inhibition of key enzymes linked to hypertension and type 2 diabetes. Food Biosci. 2020, 35, 100577. [Google Scholar] [CrossRef]
- Dumitrascu, L.; Stanciuc, N.; Borda, D.; Neagu, C.; Enachi, E.; Barbu, V.; Aprodu, I. Microencapsulation of bioactive compounds from cornelian cherry fruits using different biopolymers with soy proteins. Food Biosci. 2021, 41, 101032. [Google Scholar] [CrossRef]
- Sobel, R.; Versic, R.; Gaonkar, A.G. Introduction to Microencapsulation and Controlled Delivery in Foods. In Microencapsulation in the Food Industry, 2nd ed.; Elver Science: Amsterdam, The Netherlands, 2014; pp. 3–12. [Google Scholar]
- Zimmermann-Stein, K.; Ruiz-Espinoza, H. Estructura y funcionalidad de proteínas lácteas: Efecto de modificaciones inducidas por medios físicos, químicos y enzimáticos. Temas Sel. De Ing. De Aliment. 2010, 4, 24–37. Available online: https://tsia.udlap.mx/estructura-y-funcionalidad-de-proteinas-lacteas-efecto-de-modificaciones-inducidas-por-medios-fisicos-quimicos-y-enzimaticos/ (accessed on 22 September 2021).



| Factor 1: Maltodextrin Supplementary Wall Material | Factor 2: Concentration of Maltodextrin Supplementary Wall Material | |
|---|---|---|
| 10 wt% | 15 wt% | |
| Sodium caseinate | F1 | F2 |
| Soy protein | F3 | F4 |
| Compound | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Maltodextrin | 15.41 1 | 15.41 | 14.55 | 14.55 |
| Sodium Caseinate | 1.71 | 0 | 2.57 | 0 |
| Soy Protein | 0 | 1.71 | 0 | 2.57 |
| Total dissolved solids (wall material) | 17.12 | 17.12 | 17.12 | 17.12 |
| Water | 74.32 | 74.32 | 74.32 | 74.32 |
| Peppermint | 4.28 | 4.28 | 4.28 | 4.28 |
| Chamomile | 4.28 | 4.28 | 4.28 | 4.28 |
| Total aqueous phase | 82.88 | 82.88 | 82.88 | 82.88 |
| Total mix | 100 | 100 | 100 | 100 |
| Formulation | Wall Material | wt% | Circularity | Feret’s Diameter (μm) |
|---|---|---|---|---|
| F1 | MD + CS | 10 | 0.927 a 2 (0.012) 1 | 13.87 c (0.68) |
| F2 | MD + PS | 10 | 0.926 a (0.011) | 15.89 b,c (0.79) |
| F3 | MD + CS | 15 | 0.878 b (0.024) | 19.23 a (2.003) |
| F4 | MD + PS | 15 | 0.895 b (0.012) | 17.88 a,b (1.16) |
| Formulation | Wall Material | wt% | Entropy | IDM | FDt | Correlation | Contrast | ASM |
|---|---|---|---|---|---|---|---|---|
| F1 | MD + CS | 10 | 7.60 a,b 1 (0.35) 2 | 0.156 a,b (0.022) | 2.52 a (0.03) | 2.00·10−3 a (9.4·10−4) | 182.385 a (18.78) | 6.38·10−4 a,b (2.05·10−4) |
| F2 | MD + PS | 10 | 7.33 b (0.41) | 0.172 a (0.027) | 2.53 a (0.14) | 2.33·10−3 a (8.6·10−4) | 158.923 a,b (30.69) | 8.51·10−4 a (2.49·10−4) |
| F3 | MD + CS | 15 | 7.87 a (0.28) | 0.151 a,b (0.031) | 2.50 a (0.06) | 1.67·10−3 a (5.4·10−4) | 136.645 b (33.26) | 5.21·10−4 b (1.55·10−4) |
| F4 | MD + PS | 15 | 7.84 a,b (0.26) | 0.133 b (0.015) | 2.50 a (0.02) | 1.99·10−3 a (6.8·10−4) | 193.138 a (13.25) | 5.44·10−4 b (1.68·10−4) |
| Formulation | Wall Material | wt% | Solubility (%) | Moisture (%) | Bulk Density (g/mL) |
|---|---|---|---|---|---|
| F1 | MD + CS | 10 | 97.73 b 2 (0.76) 1 | 2.69 a (0.53) | 0.250 b (0.020) |
| F2 | MD + PS | 10 | 98.01 a,b (0.50) | 2.71 a (0.21) | 0.302 a (0.046) |
| F3 | MD + CS | 15 | 97.99 a,b (0.20) | 3.11 a (1.41) | 0.248 b (0.07) |
| F4 | MD + PS | 15 | 98.29 b (0.29) | 2.78 a (1.28) | 0.291 a,b (0.038) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Flórez, L.S.; Cabrera-Rodríguez, D.; Hernández-Carrión, M. Encapsulation of Menthol and Luteolin Using Hydrocolloids as Wall Material to Formulate Instant Aromatic Beverages. Foods 2023, 12, 2080. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12102080
Mora-Flórez LS, Cabrera-Rodríguez D, Hernández-Carrión M. Encapsulation of Menthol and Luteolin Using Hydrocolloids as Wall Material to Formulate Instant Aromatic Beverages. Foods. 2023; 12(10):2080. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12102080
Chicago/Turabian StyleMora-Flórez, Laura Sofía, Daniel Cabrera-Rodríguez, and María Hernández-Carrión. 2023. "Encapsulation of Menthol and Luteolin Using Hydrocolloids as Wall Material to Formulate Instant Aromatic Beverages" Foods 12, no. 10: 2080. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12102080