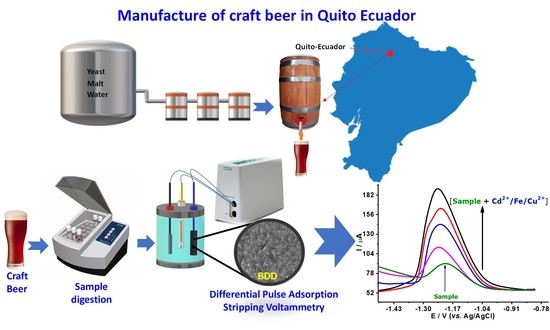
Electrochemical Evaluation of Cd, Cu, and Fe in Different Brands of Craft Beers from Quito, Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Equipment, Reagents, and Samples
2.1.1. Materials and Equipment
2.1.2. Reagents
2.1.3. Samples
2.2. Characterization of the Boron-Doped Diamond Electrode by Scanning Electron Microscope and Electrochemically Using the K3[Fe(CN)6]/K4[Fe(CN)6] Redox System
2.3. Preparation of the Craft Beer Sampler
2.4. Procedure for the Evaluation of Cd, Cu, and Fe by Differential Pulse Adsorption Stripping Voltammetry
2.4.1. Selection of Electrolyte Support
2.4.2. Determination of Optimal Differential Pulse Adsorption Stripping Voltammetry Parameters
2.4.3. Construction of the Calibration Plot and Determination of the Detection and Quantification Limit
2.4.4. Search for Optimal Differential Pulse Adsorption Stripping Voltammetry Parameters in the Craft Beer Sample
2.4.5. Standard Addition Plot
2.4.6. Evaluation of Repeatability, Reproducibility, and Recovery in the Determination of Cd, Cu, and Fe by Differential Pulse Adsorption Stripping Voltammetry
2.5. Determination of the Concentration of Heavy Metals Cd, Cu, and Fe in Craft Beers Using Differential Pulse Adsorption Stripping Voltammetry
2.6. Determination of the Concentration of Heavy Metals Cd, Cu, and Fe in Craft Beers by Flame Atomic Absorption Spectrometry
3. Results
3.1. Microscopic Characterization of the Boron-Doped Diamond Electrode
3.2. Electrochemical Characterization of the Boron-Doped Diamond Electrode
3.3. Determination of Cd(II), Cu(II), and Fe(III) by Differential Pulse Adsorption Stripping Voltammetry at Boron-Doped Diamond Electrode
3.3.1. Selection of Supporting Electrolyte for the Determination of Cd(II), Cu(II), and Fe(III) by Differential Pulse Adsorption Stripping Voltammetry
3.3.2. Optimal Differential Pulse Adsorption Stripping Voltammetry Parameters for the Determination of Cd(II), Cu(II), and Fe(III)
3.3.3. Calibration Curve, Limit of Detection, and Limit of Quantification in the Determination of Cd(II), Cu(II), and Fe(III)
3.3.4. Optimal Parameters of Differential Pulse Adsorption Stripping Voltammetry for the Determination of Cd, Cu, and Fe with Food Matrix Effect
3.3.5. Standard Addition Plot of Cd(II), Cu(II), and Fe(III)
3.3.6. Repeatability, Reproducibility, and Recovery in the Determination of Cd, Cu, and Fe
3.4. Results of Concentration of Cd, Cu, and Fe in Craft Beers
Comparison of the Results Obtained between DPASV and FAAS in the Determination of Cd, Cu, and Fe
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvillo, E. La Cerveza Artesanal, una Experiencia Multisensorial. Available online: https://www2.deloitte.com/content/dam/Deloitte/mx/Documents/consumer-business/2017/Cerveza-Artesanal-Mexico-2017.pdf (accessed on 20 March 2020).
- Fuentes, E. Las Cervezas Artesanales Embriagan a Latinoamérica. Available online: https://alnavio.es/las-cervezas-artesanales-embriagan-a-latinoamerica/ (accessed on 10 April 2023).
- Quintana Lombeida, M.D.; Aguilar Herrera, J. Evaluación de las cervezas artesanales de producción nacional y su maridaje con la cocina ecuatoriana. INNOVA Res. J. 2018, 3, 332–346. [Google Scholar] [CrossRef]
- Ciont, C.; Epuran, A.; Kerezsi, A.D.; Coldea, T.E.; Mudura, E.; Pasqualone, A.; Zhao, H.; Suharoschi, R.; Vriesekoop, F.; Pop, O.L. Beer Safety: New Challenges and Future Trends within Craft and Large-Scale Production. Foods 2022, 11, 2693. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, T.; Rastelli, S.; Mulazzi, A.; Donadini, G.; Pietri, A. Known and Emerging Mycotoxins in Small- and Large-Scale Brewed Beer. Beverages 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Gigliarelli, P. Noventa por Ciento Agua. Revista MASH-Ciencia Cervecera. Available online: https://www.revistamash.com/2017/detalle.php?id=406 (accessed on 14 May 2020).
- Murillo-Godínez, G. Hemocromatosis. Med. Int. Méx 2019, 35, 896–905. [Google Scholar] [CrossRef]
- Yan, L.-J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Feoktistova Victorava, L.; Clark Feoktistova, Y. El metabolismo del cobre. Sus consecuencias para la salud humana Metabolism of copper. Its consequences for human health. Medisur 2018, 6, 579–587. [Google Scholar]
- Maddela, N.R.; Kakarla, D.; García, L.C.; Chakraborty, S.; Venkateswarlu, K.; Megharaj, M. Cocoa-Laden Cadmium Threatens Human Health and Cacao Economy: A Critical View. Sci. Total. Environ. 2020, 720, 137645. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, Y.; Li, Y.; Zhao, H.; Wang, X.; Shen, Y.; Kuang, X. Correlation between Environmental Low-Dose Cadmium Exposure and Early Kidney Damage: A Comparative Study in an Industrial Zone vs. a Living Quarter in Shanghai, China. Environ. Toxicol. Pharmacol. 2020, 79, 103381. [Google Scholar] [CrossRef]
- Ribeiro-Filho, N.; Linforth, R.; Powell, C.D.; Fisk, I.D. Influence of Essential Inorganic Elements on Flavour Formation during Yeast Fermentation. Food Chem. 2021, 361, 130025. [Google Scholar] [CrossRef]
- Jenkins, D.; James, S.; Dehrmann, F.; Smart, K.; Cook, D. Impacts of Copper, Iron, and Manganese Metal Ions on the EPR Assessment of Beer Oxidative Stability. J. Am. Soc. Brew. Chem. 2018, 76, 50–57. [Google Scholar] [CrossRef]
- Van Mieghem, T.; Delvaux, F.; Dekleermaeker, S.; Britton, S.J. Top of the Ferrous Wheel—The Influence of Iron Ions on Flavor Deterioration in Beer. J. Am. Soc. Brew. Chem. 2022, 1–11. [Google Scholar] [CrossRef]
- Wall-Martínez, H.A.; Pascari, X.; Ramos, A.J.; Marín, S.; Sanchis, V. Frequency and Levels of Mycotoxins in Beer from the Mexican Market and Exposure Estimate for Deoxynivalenol Mycotoxins. Mycotoxin Res. 2019, 35, 207–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, H.E.; Santos, I.C.; Hildenbrand, Z.L.; Schug, K.A. A Review of the Analytical Methods Used for Beer Ingredient and Finished Product Analysis and Quality Control. Anal. Chim. Acta 2019, 1085, 1–20. [Google Scholar] [CrossRef] [PubMed]
- He, N.X.; Bayen, S. An Overview of Chemical Contaminants and Other Undesirable Chemicals in Alcoholic Beverages and Strategies for Analysis. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3916–3950. [Google Scholar] [CrossRef]
- Pelegrín, C.J.; Flores, Y.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Analysis of Chemical Contaminants in Beverages. Beverages 2020, 6, 32. [Google Scholar] [CrossRef]
- Amorello, D.; Barreca, S.; Gulli, E.; Orecchio, S. Platinum and Rhodium in Wine Samples by Using Voltammetric Techniques. Microchem. J. 2017, 130, 229–235. [Google Scholar] [CrossRef]
- García-Vítores, M.E.G.; Rincon, D.S. Determinación Voltamperométrica de Hierro en Vinos. Master’s Thesis, Universidad de Valladolid, Palencia, Spain, 2017. [Google Scholar]
- Deng, P.; Xiao, J.; Feng, J.; Tian, Y.; Wu, Y.; Li, J.; He, Q. Highly Sensitive Electrochemical Sensor for Tyrosine Detection Using a Sub-Millimeter Electrode. Microchem. J. 2021, 165, 106106. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Mohammad-Rezaei, R.; Salimi, A. Preconcentration of Mercury(II) Using a Magnetite@carbon/Dithizone Nanocomposite, and Its Quantification by Anodic Stripping Voltammetry. Microchim. Acta 2020, 187, 2. [Google Scholar] [CrossRef]
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the Practicalities of Anodic Stripping Voltammetry for Heavy Metal Detection: A Tutorial Review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef]
- Wygant, B.R.; Lambert, T.N. Thin Film Electrodes for Anodic Stripping Voltammetry: A Mini-Review. Front. Chem. 2022, 9, 809535. [Google Scholar] [CrossRef] [PubMed]
- Becerra Delgado, J.M. Determinación de Metales en Cervezas; Universidad del Azuay: Quito, Ecuador, 2014. [Google Scholar]
- Sadeghi, N.; Jodakhanlou, M.; Oveisi, M.R.; Jannat, B.; Behzad, M.; Hajimahmoodi, M. Determination of Zinc and Copper Micronutrients and Lead and Cadmium Contaminants in Non-Alcoholic Malt Beverages by Anodic Stripping Voltammetry. J. Food Safe Hyg. 2017, 3, 6. [Google Scholar]
- Sancho, D.; Blanco, C.A.; Caballero, I.; Pascual, A. Free Iron in Pale, Dark and Alcohol-Free Commercial Lager Beers: Free Iron in Commercial Lager Beers. J. Sci. Food Agric. 2011, 91, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Borja Vera, R.A. Determinación de Pb(II), Zn(II) en Cervezas Artesanales de Quito, Mediante Voltametría de Redisolución Anódica; Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2019. [Google Scholar]
- Loaiza, D. Determinación de Cd(II) y As(III) en Partículas de Hollín de Motor de Combustión Interna Mediante Voltamperometría de Redisolución Anódica. Grado; Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2020. [Google Scholar]
- Yence, M.; Cetinkaya, A.; Ozcelikay, G.; Kaya, S.I.; Ozkan, S.A. Boron-Doped Diamond Electrodes: Recent Developments and Advances in View of Electrochemical Drug Sensors. Crit. Rev. Anal. Chem. 2022, 52, 1122–1138. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, K.; Sun, J.; Fereja, T.H.; Lan, Y.; Zhang, W.; Xu, G. Boron-Doped Diamond: Current Progress and Challenges in View of Electroanalytical Applications. Anal. Methods 2019, 11, 397–414. [Google Scholar] [CrossRef]
- INEN. Instituto Ecuatoriano de Normalización NTE INEN 2262. Bebidas alcohólicas. Requisitos. Available online: https://www.normalizacion.gob.ec/buzon/normas/nte_inen_2262-1.pdf (accessed on 17 February 2020).
- BRASIL. Ministério da Saúde Decreto 55.871/65 de 26/03/. In Compêndio da Legislação de Alimentos—Consolidação das Normas e Padrões de Alimentos, Vol. I (Atos do Ministério da Saúde); Revisão no.7; Associação Brasileira das Indústrias de Alimentos: São Paulo, Brazil, 1999. [Google Scholar]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Am. Chem. Soc. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Ogunfowokan, A.O.; Adekunle, A.S.; Oyebode, B.A.; Oyekunle, J.A.O.; Komolafe, A.O.; Omoniyi-Esan, G.O. Determination of Heavy Metals in Urine of Patients and Tissue of Corpses by Atomic Absorption Spectroscopy. Chem. Afr. 2019, 2, 699–712. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, J.V. A Practical Guide to Using Boron Doped Diamond in Electrochemical Research. Phys. Chem. Chem. Phys. 2015, 17, 2935–2949. [Google Scholar] [CrossRef]
- Xu, J.; Einaga, Y. Effect of Sp2 Species in a Boron-Doped Diamond Electrode on the Electrochemical Reduction of CO2. Electrochem. Commun. 2020, 115, 106731. [Google Scholar] [CrossRef]
- Agustiany, T.; Khalil, M.; Einaga, Y.; Jiwanti, P.K.; Ivandini, T.A. Stable Iridium-Modified Boron-Doped Diamond Electrode for the Application in Electrochemical Detection of Arsenic (III). Mater. Chem. Phys. 2020, 244, 122723. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Ficek, M.; Malinowska, N.; Gupta, S.; Meek, R.; Niedziałkowski, P.; Rycewicz, M.; Sawczak, M.; Ryl, J.; Ossowski, T. Electrochemical Performance of Thin Free-Standing Boron-Doped Diamond Nanosheet Electrodes. J. Electroanal. Chem. 2020, 862, 114016. [Google Scholar] [CrossRef]
- González-Hernández, J.; Alvarado-Gámez, A.L.; Arroyo-Mora, L.E.; Barquero-Quirós, M. Electrochemical Determination of Novel Psychoactive Substances by Differential Pulse Voltammetry Using a Microcell for Boron-Doped Diamond Electrode and Screen-Printed Electrodes Based on Carbon and Platinum. J. Electroanal. Chem. 2021, 882, 114994. [Google Scholar] [CrossRef]
- Ornelas Dávila, O.; Lacalle Bergeron, L.; Ruiz Gutiérrez, P.; Dávila Jiménez, M.M.; Sirés, I.; Brillas, E.; Roig Navarro, A.F.; Beltrán Arandes, J.; Sancho Llopis, J.V. Electrochemical Oxidation of Dibenzothiophene Compounds on BDD Electrode in Acetonitrile–Water Medium. J. Electroanal. Chem. 2019, 847, 113172. [Google Scholar] [CrossRef]
- Einaga, Y. Boron-Doped Diamond Electrodes: Fundamentals for Electrochemical Applications. Acc. Chem. Res. 2022, 55, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Kamoshida, N.; Kasahara, S.; Ikemiya, N.; Hoshi, N.; Nakamura, M.; Einaga, Y. In Situ ATR-IR Study of Fe(CN)63−/Fe(CN)64− Redox System on Boron-Doped Diamond Electrode. Diam. Relat. Mater. 2019, 93, 50–53. [Google Scholar] [CrossRef]
- Kim, D.Y.; Yang, J.C.; Kim, H.W.; Swain, G.M. Heterogeneous Electron-Transfer Rate Constants for Ferrocene and Ferrocene Carboxylic Acid at Boron-Doped Diamond Electrodes in a Room Temperature Ionic Liquid. Electrochimica Acta 2013, 94, 49–56. [Google Scholar] [CrossRef]
- Rehacek, V.; Hotovy, I.; Marton, M.; Mikolasek, M.; Michniak, P.; Vincze, A.; Kromka, A.; Vojs, M. Voltammetric Characterization of Boron-Doped Diamond Electrodes for Electroanalytical Applications. J. Electroanal. Chem. 2020, 862, 114020. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- de Souza, L.V.; da Rosa, D.S.; Tkachenko, O.S.; Gomes, A.d.A.; Costa, T.M.H.; Arenas, L.T.; Benvenutti, E.V. The Role Silica Pore Structure Plays in the Performance of Modified Carbon Paste Electrodes. Ionics 2019, 25, 3259–3268. [Google Scholar] [CrossRef]
- Sigcha-Pallo, C.; Peralta-Hernández, J.; Alulema-Pullupaxi, P.; Carrera, P.; Fernández, L.; Pozo, P.; Espinoza-Montero, P. Photoelectrocatalytic Degradation of Diclofenac with a Boron-Doped Diamond Electrode Modified with Titanium Dioxide as a Photoanode. Environ. Res. 2022, 202, 113362. [Google Scholar] [CrossRef]
- Mohammed, M.Q.; Ismail, H.K.; Alesary, H.F.; Barton, S. Use of a Schiff Base-Modified Conducting Polymer Electrode for Electrochemical Assay of Cd(II) and Pb(II) Ions by Square Wave Voltammetry. Chem. Pap. 2022, 76, 715–729. [Google Scholar] [CrossRef]
- Sawan, S.; Maalouf, R.; Errachid, A.; Jaffrezic-Renault, N. Metal and Metal Oxide Nanoparticles in the Voltammetric Detection of Heavy Metals: A Review. TrAC Trends Anal. Chem. 2020, 131, 116014. [Google Scholar] [CrossRef]
- Alam, R.; Ahmed, Z.; Howladar, M.F. Evaluation of Heavy Metal Contamination in Water, Soil and Plant around the Open Landfill Site Mogla Bazar in Sylhet, Bangladesh. Groundw. Sustain. Dev. 2020, 10, 100311. [Google Scholar] [CrossRef]
- Hui Li, A.S.; Sathishkumar, P.; Selahuddeen, M.L.; Asyraf Wan Mahmood, W.M.; Zainal Abidin, M.H.; Wahab, R.A.; Mohamed Huri, M.A.; Abdullah, F. Adverse Environmental Effects of Disposable Face Masks Due to the Excess Usage. Environ. Pollut. 2022, 308, 119674. [Google Scholar] [CrossRef] [PubMed]
- Tefera, M.; Ayele, D. Investigation of Total Polyphenol, Antioxidant Activity, and Levels of Metals in Ethiopian Commercial Beers. Cogent Chem. 2020, 6, 1824336. [Google Scholar] [CrossRef]
- Ando, T.; Asai, K.; Macpherson, J.; Einaga, Y.; Fukuma, T.; Takahashi, Y. Nanoscale Reactivity Mapping of a Single-Crystal Boron-Doped Diamond Particle. Anal. Chem. 2021, 93, 5831–5838. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, X.; Cui, Z.; Xu, C.; Sun, Z.; Li, J.; Liu, J.; Tian, Y.; Li, H. A Nanometer-Sized Graphite/Boron-Doped Diamond Electrochemical Sensor for Sensitive Detection of Acetaminophen. ACS Omega 2021, 6, 6326–6334. [Google Scholar] [CrossRef]
- Zribi, B.; Dragoe, D.; Scorsone, E. BDD Electrodes Modified with Metal Nano-Catalysts for Coffee Discrimination in Real Samples. Sens. Actuators B Chem. 2019, 290, 147–154. [Google Scholar] [CrossRef]
- Takagi, K.; Natsui, K.; Watanabe, T.; Einaga, Y. Increasing the Electric Double-Layer Capacitance in Boron-Doped Diamond Electrodes. ChemElectroChem 2019, 6, 1683–1687. [Google Scholar] [CrossRef]
- Baluchová, S.; Taylor, A.; Mortet, V.; Sedláková, S.; Klimša, L.; Kopeček, J.; Hák, O.; Schwarzová-Pecková, K. Porous Boron Doped Diamond for Dopamine Sensing: Effect of Boron Doping Level on Morphology and Electrochemical Performance. Electrochim. Acta 2019, 327, 135025. [Google Scholar] [CrossRef]
- Kabir, H.; Zhu, H.; May, J.; Hamal, K.; Kan, Y.; Williams, T.; Echeverria, E.; McIlroy, D.N.; Estrada, D.; Davis, P.H.; et al. The Sp2-Sp3 Carbon Hybridization Content of Nanocrystalline Graphite from Pyrolyzed Vegetable Oil, Comparison of Electrochemistry and Physical Properties with Other Carbon Forms and Allotropes. Carbon. 2019, 144, 831–840. [Google Scholar] [CrossRef]
- Zanello, P.; Nervi, C.; De Biani, F. Inorganic Electrochemistry: Theory, Practice and Application, 2nd ed.; Royal Society of Chemistry: London, UK, 2019; Volume 1, ISBN 0-85404-661-5. [Google Scholar]
- Allen, J.B.; Larry, R.F. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: Hoboken, FL, USA, 2001; Volume 1, ISBN 0-471-04372-9. [Google Scholar]
- Bennett, J.A.; Wang, J.; Show, Y.; Swain, G.M. Effect of Sp2-Bonded Nondiamond Carbon Impurity on the Response of Boron-Doped Polycrystalline Diamond Thin-Film Electrodes. J. Electrochem. Soc. 2004, 151, E306. [Google Scholar] [CrossRef]
- González-Meza, O.A.; Larios-Durán, E.R.; Gutiérrez-Becerra, A.; Casillas, N.; Escalante, J.I.; Bárcena-Soto, M. Development of a Randles-Ševčík-like Equation to Predict the Peak Current of Cyclic Voltammetry for Solid Metal Hexacyanoferrates. J. Solid. State Electrochem. 2019, 23, 3123–3133. [Google Scholar] [CrossRef]
- Ryl, J.; Burczyk, L.; Zielinski, A.; Ficek, M.; Franczak, A.; Bogdanowicz, R.; Darowicki, K. Heterogeneous Oxidation of Highly Boron-Doped Diamond Electrodes and Its Influence on the Surface Distribution of Electrochemical Activity. Electrochim. Acta 2019, 297, 1018–1027. [Google Scholar] [CrossRef]
- Komkova, M.A.; Pasquarelli, A.; Andreev, E.A.; Galushin, A.A.; Karyakin, A.A. Prussian Blue Modified Boron-Doped Diamond Interfaces for Advanced H2O2 Electrochemical Sensors. Electrochim. Acta 2020, 339, 135924. [Google Scholar] [CrossRef]
- Liu, Z.; Baluchová, S.; Sartori, A.F.; Li, Z.; Gonzalez-Garcia, Y.; Schreck, M.; Buijnsters, J.G. Heavily Boron-Doped Diamond Grown on Scalable Heteroepitaxial Quasi-Substrates: A Promising Single Crystal Material for Electrochemical Sensing Applications. Carbon 2023, 201, 1229–1240. [Google Scholar] [CrossRef]
- Hamal, K.; May, J.; Zhu, H.; Dalbec, F.; Echeverria, E.; McIlroy, D.N.; Aston, E.; Cheng, I.F. Electrochemical Aspects of a Nitrogen-Doped Pseudo-Graphitic Carbon Material: Resistance to Electrode Fouling by Air-Aging and Dopamine Electro-Oxidation. C 2020, 6, 68. [Google Scholar] [CrossRef]
- Yang, X. Electrical and Electrochemical Properties of Nitrogen-Containing Tetrahedral Amorphous Carbon (Ta-C) Thin Films. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2013. [Google Scholar]
- Wang, R.; Ji, W.; Huang, L.; Guo, L.; Wang, X. Electrochemical Determination of Lead(II) in Environmental Waters Using a Sulfydryl Modified Covalent Organic Framework by Square Wave Anodic Stripping Voltammetry (SWASV). Anal. Lett. 2019, 52, 1757–1770. [Google Scholar] [CrossRef]
- Passaghe, P.; Bertoli, S.; Tubaro, F.; Buiatti, S. Monitoring of Some Selected Heavy Metals throughout the Brewing Process of Craft Beers by Inductively Coupled Plasma Mass Spectrometry. Eur. Food Res. Technol. 2015, 241, 199–215. [Google Scholar] [CrossRef]
- Bonilla Medina, C. Evaluación de las Características de Desempeño de un Método Analítico para la Determinación de Hierro Total en Agua de Consumo Humano Mediante Espectrofotometría Ultravioleta. Ph.D. Thesis, Universidad de San Carlos de Guatemala, San Carlos, Guatemala, 2022. [Google Scholar]
- Marcano, E.; Gómez, C.; Benzo, Z.; Laine, J. Estudio preliminar sobre la determinación de elementos traza en cervezas venezolanas por ICP-OES. Quím. Nova 2010, 33, 653–655. [Google Scholar] [CrossRef]
- Pérez López, E.; Alvarado Rodriguez, D.C. Cuantificación por absorción atómica de Cu, Fe y Zn en alcohol destilado y agua. UNED Res. J. 2018, 10, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Zambrzycka-Szelewa, E.; Nalewajko-Sieliwoniuk, E.; Zaremba, M.; Bajguz, A.; Godlewska-Żyłkiewicz, B. The Mineral Profile of Polish Beers by Fast Sequential Multielement HR CS FAAS Analysis and Its Correlation with Total Phenolic Content and Antioxidant Activity by Chemometric Methods. Molecules 2020, 25, 3402. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron Deficiency Anaemia Revisited. J. Intern. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muros, J.J.; Cabrera-Vique, C.; Briones, M.; Seiquer, I. Assessing the Dietary Intake of Calcium, Magnesium, Iron, Zinc and Copper in Institutionalised Children and Adolescents from Guatemala. Contribution of Nutritional Supplements. J. Trace Elem. Med. Biol. 2019, 53, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ho-Wang, Y. National Center for Biotechnology Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK459224/ (accessed on 15 April 2023).
- Silva, L.R.G.; Mutz, Y.S.; Stefano, J.S.; Conte-Junior, C.A.; Ferreira, R.d.Q. A Simple and Reliable Electroanalytical Method Employing a Disposable Commercial Electrode for Simultaneous Determination of Lead(II) and Mercury(II) in Beer. J. Food Compos. Anal. 2022, 110, 104564. [Google Scholar] [CrossRef]









| Beer Code | Origin | Sector of Sale a | Specific Ingredients b | Characteristic c |
|---|---|---|---|---|
| CAR-A | Germany | Diego de Almagro | Caramel barley malt, roasted barley, brown sugar | IPA, 7.0 Vol% |
| CAR-B | Ecuador | Foch | Caramel barley malt | Ale, 5.0 Vol% |
| CAR-C | Ecuador | Avenue 12 de Octubre | Honey bee | Ale, 6.5 Vol% |
| CAR-D | Scotland | Pradera | Roasted barley, roasted hazelnuts | Ale, 6.0 Vol% |
| CAR-E | Ecuador | Avenue Orellana | Caramel barley malt, roasted barley, | Ale, 7.0 Vol% |
| CAR-F | Ecuador | Río Coca | Natural blackberry flavoring | Ale, 6.0 Vol% |
| CAR-G | Ireland | Avenue Oswaldo Guayasamin | Caramel barley malt, roasted barley | Ale, 7.0 Vol% |
| CAR-H | Ecuador | Avenue Whymper | Caramelized malt, roasted barley | Ale, 5.7Vol% |
| CAR-I | Ecuador | Avenue Mariana de Jesús | Caramel barley malt | Ale, 6.0 Vol% |
| CAR-J | Ecuador | Avenue 6 de Diciembre | Cinnamon, vanilla | Ale, 5.0 Vol% |
| CAR-K | Ecuador | Avenue Vaca de Castro | Caramelized malt, Blackberry, cocoa, cinnamon | Ale, 7.0 Vol% |
| CAR-L | Ecuador | Bicentenario | Ale, 5.0 Vol% | |
| CAR-M | Ecuador | Reina Victoria | Caramelized malt, Cotton candy flavoring | Ale, 6.9 Vol% |
| Metal | Slope of the Curve μA (μg/L)−1 | Correlation Coefficient, r | Determination Coefficient, R2 | DL μg L−1 | QL μg L−1 |
|---|---|---|---|---|---|
| Cd(II) | 0.62665 | 0.999 | 0.998 | 6.31 | 21.04 |
| Cu(II) | 10.9618 | 0.998 | 0.994 | 1.76 | 5.87 |
| Fe(III) | 6.71163 | 0.999 | 0.997 | 1.72 | 5.72 |
| Metal | Slope of the Plot μA (μg/L)−1 | Correlation Coefficient, r | Determination Coefficient, R2 | |||
|---|---|---|---|---|---|---|
| No Matrix Effect | With Matrix Effect | No Matrix Effect | With Matrix Effect | No Matrix Effect | With Matrix Effect | |
| Cd(II) | 0.62665 | 10.004 | 0.999 | 0.994 | 0.98 | 0.984 |
| Cu(II) | 10.9618 | 59.7068 | 0.998 | 0.997 | 0.994 | 0.991 |
| Fe(III) | 6.71163 | 29.7239 | 0.999 | 0.993 | 0.997 | 0.984 |
| Heavy Metal | Days | (RSD%) | Repeatability (RSD%) | Reproducibility (RSD%) |
|---|---|---|---|---|
| Cd(II) | 1 | 1.06 | 1.06 | 1.61 |
| 2 | 1.52 | |||
| 3 | 2.23 | |||
| Cu(II) | 1 | 2.43 | 2.43 | 2.94 |
| 2 | 2.89 | |||
| 3 | 3.49 | |||
| Fe(III) | 1 | 1.34 | 1.34 | 1.83 |
| 2 | 1.79 | |||
| 3 | 2.37 |
| Code of Beer | Heavy Metal | Average (mg L−1) | Sd | RSD% | Complies with Food Standards * |
|---|---|---|---|---|---|
| CAR-A | Cd | 0.0589 | 0.0262 | 4.44 | Yes |
| Cu | 0.3015 | 0.0312 | 10.36 | Yes | |
| Fe | 0.2425 | 0.0168 | 6.92 | No | |
| CAR-B | Cd | 0.0378 | 0.0496 | 13.12 | Yes |
| Cu | 0.3692 | 0.0190 | 5.15 | Yes | |
| Fe | 0.1851 | 0.0111 | 5.98 | Yes | |
| CAR-C | Cd | 0.0326 | 0.0010 | 3.10 | Yes |
| Cu | 0.4495 | 0.0240 | 5.34 | Yes | |
| Fe | 0.1677 | 0.0024 | 1.41 | Yes | |
| CAR-D | Cd | 0.0313 | 0.0431 | 13.75 | Yes |
| Cu | 0.1915 | 0.0067 | 3.50 | Yes | |
| Fe | 0.1556 | 0.0046 | 2.93 | Yes | |
| CAR-E | Cd | 0.0449 | 0.0297 | 6.62 | Yes |
| Cu | 0.2541 | 0.0165 | 6.51 | Yes | |
| Fe | 0.3159 | 0.0102 | 3.22 | No | |
| CAR-F | Cd | 0.0211 | 0.0178 | 8.42 | Yes |
| Cu | 0.1937 | 0.0162 | 8.34 | Yes | |
| Fe | 0.1613 | 0.0047 | 2.88 | Yes | |
| CAR-G | Cd | 0.0720 | 0.0587 | 8.15 | Yes |
| Cu | 0.2661 | 0.0123 | 4.61 | Yes | |
| Fe | 0.1530 | 0.0037 | 2.44 | Yes | |
| CAR-H | Cd | 0.0191 | 0.0064 | 3.36 | Yes |
| Cu | 0.3207 | 0.0168 | 5.25 | Yes | |
| Fe | 0.2920 | 0.0098 | 3.35 | No | |
| CAR-I | Cd | 0.0083 | 0.0096 | 11.64 | Yes |
| Cu | 0.4660 | 0.0364 | 7.82 | Yes | |
| Fe | 0.1250 | 0.0003 | 0.21 | Yes | |
| CAR-J | Cd | 0.237 | 0.0286 | 12.07 | Yes |
| Cu | 0.2393 | 0.0259 | 10.83 | Yes | |
| Fe | 0.1309 | 0.0018 | 1.34 | Yes | |
| CAR-K | Cd | 0.0811 | 0.0676 | 8.34 | Yes |
| Cu | 0.4621 | 0.0310 | 6.71 | Yes | |
| Fe | 0.2663 | 0.0197 | 7.39 | No | |
| CAR-L | Cd | 0.0315 | 0.0010 | 3.20 | Yes |
| Cu | 0.1339 | 0.0110 | 8.20 | Yes | |
| Fe | 0.1869 | 0.0024 | 1.30 | Yes | |
| CAR-M | Cd | 0.0910 | 0.0651 | 7.15 | Yes |
| Cu | 0.3337 | 0.0221 | 6.61 | Yes | |
| Fe | 0.3433 | 0.0109 | 3.17 | No |
| Beer Code | Cd | Cu | Fe | |||
|---|---|---|---|---|---|---|
| DPASV (mg L−1) | FAAS (mg L−1) | DPASV (mg L−1) | FAAS (mg L−1) | DPASV (mg L−1) | FAAS (mg L−1) | |
| CAR-A | 0.0589 | 0.0335 | 0.3015 | 0.3931 | 0.2425 | ND * |
| CAR-B | 0.0378 | 0.0281 | 0.3692 | 0.2264 | 0.1851 | ND |
| CAR-C | 0.0326 | 0.0366 | 0.4495 | 0.3732 | 0.1677 | ND |
| CAR-D | 0.0313 | 0.0270 | 0.1915 | 0.1156 | 0.1556 | ND |
| CAR-E | 0.0449 | 0.0320 | 0.2541 | 0.2837 | 0.3159 | ND |
| CAR-F | 0.0211 | 0.0233 | 0.1937 | 0.2153 | 0.1613 | ND |
| CAR-G | 0.0720 | 0.0553 | 0.2661 | 0.1354 | 0.1530 | ND |
| CAR-H | 0.0191 | 0.0224 | 0.3207 | 0.2860 | 0.2920 | ND |
| CAR-I | 0.0083 | 0.0137 | 0.4660 | 0.3947 | 0.1250 | ND |
| CAR-J | 0.0237 | 0.0236 | 0.2393 | 0.1764 | 0.1309 | ND |
| CAR-K | 0.0811 | 0.0664 | 0.4621 | 0.5175 | 0.2663 | ND |
| CAR-L | 0.0315 | 0.0384 | 0.1339 | 0.0839 | 0.1869 | ND |
| CAR-M | 0.0910 | 0.0736 | 0.3337 | 0.3555 | 0.3770 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Balladares, O.; Espinoza-Montero, P.J.; Fernández, L. Electrochemical Evaluation of Cd, Cu, and Fe in Different Brands of Craft Beers from Quito, Ecuador. Foods 2023, 12, 2264. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12112264
López-Balladares O, Espinoza-Montero PJ, Fernández L. Electrochemical Evaluation of Cd, Cu, and Fe in Different Brands of Craft Beers from Quito, Ecuador. Foods. 2023; 12(11):2264. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12112264
Chicago/Turabian StyleLópez-Balladares, Oscar, Patricio J. Espinoza-Montero, and Lenys Fernández. 2023. "Electrochemical Evaluation of Cd, Cu, and Fe in Different Brands of Craft Beers from Quito, Ecuador" Foods 12, no. 11: 2264. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12112264