Construction and In Vitro Digestibility of Recrystallized Starch Encapsulated in Calcium Alginate Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RS3
2.3. Preparation of Calcium Alginate Encapsulated RS3 Gel Beads
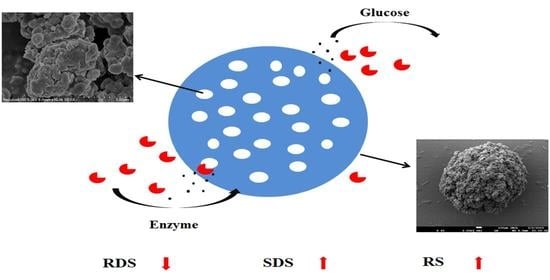
2.4. Morphological Characteristics of Beads
2.5. Texture Profile Analysis of Beads
2.6. Swelling Behavior Measurements of the Beads
2.7. Determination of Total Starch Content
2.8. In Vitro Digestibility of Beads
2.9. Statistical Analysis
3. Results and Discussion
3.1. Morphology of RS3
3.2. Morphology of Calcium Alginate Encapsulated RS3 Beads
3.3. Gel Texture Properties
3.4. Swelling Power (SP) and Solubility (S)
3.5. Determination of Total Starch Content
3.6. Determination of In Vitro Digestibility
3.7. Hydrolysis Curve
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33–50. [Google Scholar]
- Wang, M.W.; Chen, X.Y.; Zhou, L.Y.; Li, Y.; Yang, J.; Ji, N.; Xiong, L.; Sun, Q.J. Prebiotic effects of resistant starch nanoparticles on growth and proliferation of the probiotic Lactiplantibacillus plantarum subsp. plantarum. LWT-Food Sci. Technol. 2022, 154, 112572. [Google Scholar] [CrossRef]
- Peter, A.D.; Alain, S. Resistant starch, microbiome, and precision modulation. Gut. Microbes 2021, 13, 1926842. [Google Scholar]
- Miao, T.T.; Xiong, K.; Ji, N.; Xiong, L.; Sun, C.R.; Li, X.J.; Ma, A.G.; Sun, Q.J. Resistant starch nanoparticles prepared from debranched starch by medium-temperature recrystallization. Int. J. Biol. Macromol. 2020, 155, 598–604. [Google Scholar] [CrossRef]
- Wali, J.A.; Milner, A.J.; Luk, A.W.; Pulpitel, T.J.; Dodgson, T.; Facey, H.J.; Wahl, D.; Kebede, M.A.; Senior, A.M.; Sullivan, M.A.; et al. Impact of dietary carbohydrate type and protein–carbohydrate interaction on metabolic health. Nat. Metab. 2021, 3, 810–828. [Google Scholar] [CrossRef]
- Kraithonga, S.; Wang, S.K.; Junejo, S.A.; Fu, X.; Theppawong, A.; Zhang, B.; Huang, Q. Type 1 resistant starch: Nutritional properties and industry applications. Food Hydrocoll. 2022, 125, 107369. [Google Scholar] [CrossRef]
- Hsein-Chih, H.W.; Anatole, S. The double-helical molecular structure of crystalline α-amylose. Carbohydr. Res. 1978, 61, 7–26. [Google Scholar] [CrossRef]
- Ring, S.G.; Gee, J.M.; Whittam, M.; Orford, P.; Johnson, I.T. Resistant starch: Its chemical form in food stuffs and effectson digestibility in vitro. Food Chem. 1988, 28, 97–109. [Google Scholar] [CrossRef]
- Eerlingen, R.C.; Broeck, V.D.; Delcour, J.A.; Slade, L.; Levine, H. Enzyme resistant starch VI: Influence of sugars on resistant starch formation. Cereal Chem. 1994, 71, 472–476. [Google Scholar]
- Frost, G.; Leeds, A.A.; Dore, C.J.; Madeiros, S.; Brading, S.; Dornhorst, A. Glycemic index as a determinant of serum HDL-cholesterol concentration. Lancet 1999, 353, 1045–1048. [Google Scholar] [CrossRef]
- Liu, S.; Reimer, M.; Ai, Y. Yongfeng. In vitro digestibility of different types of resistant starches under high-temperature cooking conditions. Food Hydrocoll. 2020, 107, 105927. [Google Scholar] [CrossRef]
- Sun, Q.J.; Li, G.; Dai, L.; Ji, N.; Xiong, L. Green preparation and characterisation of waxy maize starch nanoparticles through enzymolysis and recrystallisation. Food Chem. 2014, 162, 223–228. [Google Scholar] [CrossRef]
- Man, J.; Wang, X.J.; Li, J.Y.; Cui, X.Y.; Hua, Z.S.; Li, J.F.; Mao, Z.B.; Zhang, S.G. Intravenous Calcium Alginate Microspheres as Drug Delivery Vehicles in Acute Kidney Injury Treatment. Micromachines 2022, 13, 538. [Google Scholar] [CrossRef]
- Lozano-Vazquez, G.; Lobato-Calleros, C.; Escalona-Buendia, H.; Chavez, G.; AlvarezRamirez, J.; Vernon-Carter, E.J. Effect of the weight ratio of alginatemodified tapioca starch on the physicochemical properties and release kinetics of chlorogenic acid containing beads. Food Hydrocoll. 2015, 48, 301–311. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Z.G.; Xiao, Z.G. Preparation, physicochemical characterization and in vitro release behavior of resveratrol-loaded oxidized gellan gum/resistant starch hydrogel beads. Carbohydr. Polym. 2021, 260, 117794. [Google Scholar] [CrossRef]
- Li, W.; Cao, F.; Fan, J.; Ouyang, S.; Luo, Q.; Zheng, J.; Zhang, G. Physically modified common buckwheat starch and their physicochemical and structural properties. Food Hydrocoll. 2014, 40, 237–244. [Google Scholar] [CrossRef]
- Giuberti, G.; Gallo, A. Reducing the glycaemic index and increasing the slowly digestible starch content in gluten-free cereal-based foods: A review. Int. J. Food Sci. Technol. 2017, 53, 50–60. [Google Scholar] [CrossRef]
- Cui, C.; Li, M.; Ji, N.; Qin, Y.; Shi, R.; Qiao, Y.; Xiong, L.; Dai, L.; Sun, Q. Calcium alginate/curdlan/corn starch@calcium alginate macrocapsules for slowly digestible and resistant starch. Carbohydr. Polym. 2022, 285, 119259. [Google Scholar] [CrossRef]
- Borczak, B.; Sikora, E.; Sikora, M.; Kapusta-Duch, J.; Rosell, C.M. Starch digestibility index and antioxidative properties of partially baked wheat-flour bakery with an addition of dietary fibre. Starch Starke 2015, 67, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Dhital, S.; Shrestha, A.K.; Gidley, M.J. Effect of cryo-milling on starches: Functionality and digestibility. Food Hydrocoll. 2010, 24, 152–163. [Google Scholar] [CrossRef]
- Hung, P.V.; Binh, V.T.; Nhi, P.H.Y.; Phi, N.T.L. Effect of heat-moisture treatment of unpolished red rice on its starch properties and in vitro digestibility. Int. J. Biol. Macromol. 2020, 154, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alhussain, M.H.; Almousa, A.; Alhowikan, A. Impact of resistant starch type-3 on glucose metabolism and appetite in healthy males. Proc. Nutr. Soc. 2021, 80, E66. [Google Scholar] [CrossRef]
- Zafeiri, I.; Beri, A.; Linter, B.; Norton, I. Mechanical properties of starch-filled alginate gel particles. Carbohydr. Polym. 2021, 255, 117373. [Google Scholar] [CrossRef] [PubMed]
- Feltre, G.; Silva, C.A.; Lima, G.B.; Menegalli, F.C.; Dacanal, G.C. Production of Thermal-Resistant Cornstarch-Alginate Beads by Dripping Agglomeration. Int. J. Food Eng. 2018, 14, 20170296. [Google Scholar] [CrossRef]
- Najafi-Soulari, S.; Shekarchizadeh, H.; Kadivar, M. Encapsulation optimization of lemon balm antioxidants in calcium alginate hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 1631–1644. [Google Scholar] [CrossRef]
- Cui, C.L.; Jiang, H.; Guan, M.H.; Ji, N.; Xiong, L.; Sun, Q.J. Characterization and in vitro digestibility of potato starch encapsulated in calcium alginate beads. Food Hydrocoll. 2022, 126, 107458. [Google Scholar] [CrossRef]
- Qin, K.L.; Sun, D.Y.; Wang, C.F.; Ji, N.; Dai, L.; Qin, Y.; Xiong, L.; Wang, T.; Sun, Q.J. Properties and in vitro digestibility of starch encapsulated in chitosan-sodium phytate capsules. Food Hydrocoll. 2023, 135, 108127. [Google Scholar] [CrossRef]






| Sample | Hardness (g) | Adhesiveness (g.sec) | Chewiness | Springiness |
|---|---|---|---|---|
| Calcium alginate beads | 475.37 ± 4.29 c | −4.29 ± 0.11 a | 182.36 ± 6.27 c | 0.72 ± 0.03 a |
| RS31@Alginate1 | 509.43 ± 3.34 b | −1.76 ± 0.04 b | 216.44 ± 11.73 b | 0.76 ± 0.01 a |
| RS35@Alginate1 | 582.34 ± 16.46 a | −0.46 ± 0.02 c | 234.75 ± 33.43 a | 0.82 ± 0.01 b |
| Sample | Hardness (g) | Adhesiveness (g.sec) | Chewiness | Springiness |
|---|---|---|---|---|
| Calcium alginate beads | 2764.26 ± 163.54 a | −0.05 ± 0.01 c | 1132.24 ± 4.65 b | 0.78 ± 0.01 a |
| RS31@Alginate1 | 2237.46 ± 127.35 b | −1.34 ± 0.03 b | 1056.61 ± 17.34 a | 0.83 ± 0.01 a |
| RS35@Alginate1 | 1836.31 ± 27.48 c | −3.24 ± 0.03 a | 1046.35 ± 41.27 a | 0.89 ± 0.01 a |
| Temperature (°C) | Swelling Power (g/g) | Solubility (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 55 °C | 65 °C | 75 °C | 85 °C | 95 °C | 55 °C | 65 °C | 75 °C | 85 °C | 95 °C | |
| Waxy corn starch | 10.35 ± 0.12 a | 12.54 ± 0.14 a | 34.48 ± 0.27 a | 35.57 ± 0.37 a | 43.22 ± 0.37 a | 4.4 ± 0.02 a | 6.63 ± 0.07 a | 7.36 ± 0.18 a | 9.68 ± 0.19 a | 19.26 ± 0.26 a |
| RS3 | 4.27 ± 0.21 b | 8.65 ± 0.23 b | 18.87 ± 0.29 b | 23.83 ± 0.56 b | 38.37 ± 0.42 b | 3.2 ± 0.02 b | 5.12 ± 0.03 b | 6.14 ± 0.21 b | 8.31 ± 0.13 b | 15.24 ± 0.28 b |
| RS35@Alginate1 | 3.38 ± 0.02 c | 5.21 ± 0.06 c | 9.36 ± 0.14 c | 11.57 ± 0.13 c | 13.21 ± 0.24 c | 2.31 ± 0.03 c | 4.12 ± 0.04 c | 5.24 ± 0.16 c | 7.42 ± 0.13 c | 9.56 ± 0.14 c |
| RS31@Alginate1 | 2.63 ± 0.13 d | 3.74 ± 0.13 d | 5.73 ± 0.04 d | 8.53 ± 0.17 d | 9.94 ± 0.14 d | 1.24 ± 0.02 d | 2.68 ± 0.04 d | 3.73 ± 0.13 d | 5.52 ± 0.14 d | 7.42 ± 0.21 d |
| Samples | TS(%) | Concentration of Sodium Alginate (% of 100 mL Water Solution) | Concentration of Calcium Chloride (% of 100 mL Water Solution) | Debranched Starch (% of 100 mL Water Solution) |
|---|---|---|---|---|
| Waxy corn starch | 98.57 ± 0.34 a | / | / | / |
| RS3 | 97.48 ± 0.27 a | / | / | / |
| RS35@Alginate1 | 80.62 ± 0.46 b | 2 | 1 | 10 |
| RS31@Alginate1 | 47.31 ± 0.35 c | 2 | 1 | 2 |
| Samples | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|
| Waxy corn starch | 93.43 ± 0.32 a | 5.25 ± 0.15 d | 1.32 ± 0.17 d |
| RS3 | 67.73 ± 0.24 b | 6.74 ± 0.16 c | 25.53 ± 0.39 c |
| RS35@Alginate1 | 31.87 ± 0.22 c | 7.62 ± 0.17 b | 60.51 ± 0.43 b |
| RS31@Alginate1 | 20.85 ± 0.34 d | 9.05 ± 0.33 a | 70.10 ± 0.64 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, K.; Zhang, R.; Qin, W.; Ji, N.; Qin, Y.; Dai, L.; Xiong, L.; Sun, Q. Construction and In Vitro Digestibility of Recrystallized Starch Encapsulated in Calcium Alginate Beads. Foods 2023, 12, 2379. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12122379
Qin K, Zhang R, Qin W, Ji N, Qin Y, Dai L, Xiong L, Sun Q. Construction and In Vitro Digestibility of Recrystallized Starch Encapsulated in Calcium Alginate Beads. Foods. 2023; 12(12):2379. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12122379
Chicago/Turabian StyleQin, Kaili, Rongyu Zhang, Weili Qin, Na Ji, Yang Qin, Lei Dai, Liu Xiong, and Qingjie Sun. 2023. "Construction and In Vitro Digestibility of Recrystallized Starch Encapsulated in Calcium Alginate Beads" Foods 12, no. 12: 2379. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12122379