Comparison of Nutritional Composition and Antioxidant Properties of Pulverized and Unutilized Portions of Waxy Barley
Abstract
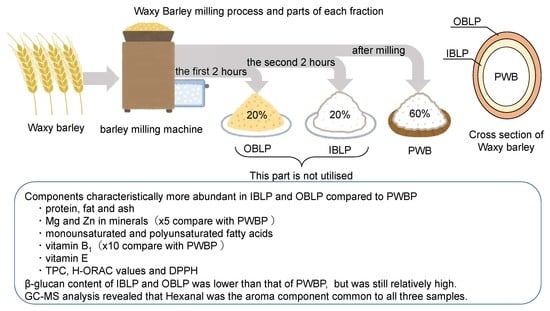
:1. Introduction
2. Materials and Methods
2.1. Materials of Barley Variety Kirarimochi
2.2. Color Tone
2.3. Scanning Electron Microscopy (SEM)
2.4. General Components, Vitamin B1, and Fatty Acids
2.5. Mineral Composition
2.6. Vitamin E (Tocopherol)
2.7. Determination of Total Polyphenol Content (TPC) and Antioxidant Activity
2.8. Beta-Glucan
2.9. GC–MS Analysis
2.10. Statistical Processing
3. Results and Discussion
3.1. Appearance, Color Tone, and SEM
3.2. General Composition
3.3. Minerals
3.4. Fatty Acid Content
3.5. β-Glucan
3.6. Vitamin B1
3.7. Vitamin E
3.8. TPC, H-ORAC, and DPPH Value
3.9. GC–MS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harding, S.V.; Storsley, J.; Thandapilly, S.J.; Ames, N.P. Lower 30 Minute Serum Insulin in Healthy Sprague-Dawley Rats Consuming Chips from Specific Barley Flour Blends. Cereal Chem. 2013, 90, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupton, J.R.; Robinson, M.C.; Morin, J.L. Cholesterol-lowering effect of barley bran flour and oil. J. Am. Diet. Assoc. 1994, 94, 65–70. [Google Scholar] [CrossRef]
- Tamagawa, K.; Iizuka, S.; Fukushima, S.; Endo, Y.; Komiyama, Y. Antioxidative activity of polyphenol extracts from barley bran. J. Jpn. Soc. Soc. Food Sci. Technol. 1997, 44, 512–515. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, G.; Jorgensen, L.; Royle, P. The potential of an insoluble dietary fiber-rich source from barley to protect from DMH-induced intestinal tumors in rats. Nutr. Canc. 1993, 19, 213–221. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.H.; Le Leu, R.K.; Royle, P.J.; Young, G.P. A comparative study of the influence of differing barley brans on DMH-induced intestinal tumours in male Sprague-Dawley rats. J. Gastroenterol. Hepatol. 1996, 11, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Tamagawa, K.; Ikeda, A.; Naganuma, K.; Wada, M.; Takita, T.; Innami, S. Effects of Different Fractions of Barley Bran on Gastrointestinal Function and Lipid Metabolism in Rats. J. Jpn. Assoc. Diet. Fiber Res. 2001, 5, 23–31. [Google Scholar]
- Theuwissen, E.; Mensink, R.P. Simultaneous intake of β-glucan and plant stanol esters affects lipid metabolism in slightly hypercholesterolemic subjects. J. Nutr. 2007, 137, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Naumann, E.; Van Rees, A.B.; Önning, G.; Öste, R.; Wydra, M.; Mensink, R.P. β-Glucan incorporated into a fruit drink effectively lowers serum LDL-cholesterol concentrations. Am. J. Clin. Nutr. 2006, 83, 601–605. [Google Scholar] [CrossRef] [Green Version]
- Demir, G.; Klein, H.; Mandel-Molinas, N.; Tuzuner, N. Beta glucan induces proliferation and activation of monocytes in peripheral blood of patients with advanced breast cancer. Int. Immunopharmacol. 2007, 7, 113–116. [Google Scholar] [CrossRef]
- Tapola, N.; Karvonen, H.; Niskanen, L.; Mikola, M.; Sarkkinen, E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 255–261. [Google Scholar] [CrossRef]
- Zhang, G.; Junmei, W.; Jinxin, C. Analysis of β-glucan content in barley cultivars from different locations of China. Food. Chem. 2002, 79, 251–254. [Google Scholar] [CrossRef]
- Ullrich, S.; Clancy, J.; Eslick, R.; Lance, R. β-Glucan content and viscosity of extracts from waxy barley. J. Cereal Sci. 1986, 4, 279–285. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Nagamine, T.; Takahashi, A.; Takayama, T.; Doi, Y.; Matsunaka, H.; Fujita, M. Breeding of Kirari-mochi: A new two-rowed waxy hull-less barley cultivar with superior quality characteristics. Breed. Sci. 2011, 61, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, K.; Komae, K.; Takahashi, A.; Yoshioka, T.; Sone, Y. Effect of waxy barley, Kirarimochi, consumption on bowel movements of late-stage elderly residents at Roken nursing home. J. Physiol. Anthropol. 2017, 36, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tsurunaga, Y.; Kanou, M.; Ikeura, H.; Makino, M.; Oowatari, Y.; Tsuchiya, I. Effect of different tea manufacturing methods on the antioxidant activity, functional components, and aroma compounds of Ocimum gratissimum. LWT 2022, 169, 114058. [Google Scholar] [CrossRef]
- The Subdivision on Resources, The Council for Science and Technology Ministry of Education, Culture, Sports, Science and Technology, Japan. Standard Tables of Food Composition in Japan; Ministry of Education, Culture, Sports, Science and Technology: Tokyo, Japan, 2020. [Google Scholar]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin−Ciocalteu assay. J. Agr. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Tsurunaga, Y.; Takahashi, T.; Katsube, T.; Kudo, A.; Kuramitsu, O.; Ishiwata, M.; Matsumoto, S. Effects of UV-B irradiation on the levels of anthocyanin, rutin and radical scavenging activity of buckwheat sprouts. Food Chem. 2013, 141, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Oki, T.; Takebayashi, J.; Yamasaki, K.; Takano-Ishikawa, Y.; Hino, A.; Yasui, A. Method validation by interlaboratory studies of improved hydrophilic oxygen radical absorbance capacity methods for the determination of antioxidant capacities of antioxidant solutions and food extracts. Anal. Sci. 2012, 28, 159. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.U.; Ko, M.J.; Chung, M.S. Hydrolysis of beta-glucan in oat flour during subcritical-water extraction. Food Chem. 2020, 308, 125670. [Google Scholar] [CrossRef]
- Farneti, B.; Cristescu, S.M.; Costa, G.; Harren, F.J.; Woltering, E.J. Rapid tomato volatile profiling by using proton-transfer reaction mass spectrometry (PTR-MS). J. Food Sci. 2012, 77, C551–C559. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.J.; Courtin, C.M.; Venema, K.; de Vries, J. Health benefits of whole grain: Effects on dietary carbohydrate quality, the gut microbiome, and consequences of processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2742–2768. [Google Scholar] [CrossRef]
- Carcea, M. Value of Wholegrain Rice in a Healthy Human Nutrition. Agriculture 2021, 11, 720. [Google Scholar] [CrossRef]
- Baik, B.K.; Ullrich, S.E. Barley for food: Characteristics, improvement, and renewed interest. J. Cereal Sci. 2008, 48, 233–242. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov./ (accessed on 12 May 2023).
- Ministry of Health, Labour and Welfare. The Dietary Reference Intakes for Japanese; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2020. [Google Scholar]
- Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey in Japan; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2019. [Google Scholar]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.M.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [Green Version]
- Bucher, H.C.; Hengstler, P.; Schindler, C.; Meier, G. N-3 polyunsaturated fatty acids in coronary heart disease: A meta-analysis of randomized controlled trials. Am. J. Med. 2002, 112, 298–304. [Google Scholar] [CrossRef]
- Brostow, D.P.; Odegaard, A.O.; Koh, W.P.; Duval, S.; Gross, M.D.; Yuan, J.M.; Pereira, M.A. Omega-3 fatty acids and incident type 2 diabetes: The Singapore Chinese Health Study. Am. J. Clin. Nutr. 2011, 94, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Folsom, A.R.; Zheng, Z.J.; Pankow, J.S.; Eckfeldt, J.H.; Investigators, A.S. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2003, 78, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Langenaeken, N.A.; Ieven, P.; Hedlund, E.G.; Kyomugasho, C.; Van De Walle, D.; Dewettinck, K.; Van Loey, A.M.; Roeffaers, M.B.J.; Courtin, C.M. Arabinoxylan, β-glucan and pectin in barley and malt endosperm cell walls: A microstructure study using CLSM and cryo-SEM. Plant J. 2020, 103, 1477–1489. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.W.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, A.M.; Titgemeier, B.; Kirkpatrick, K.; Golubic, M.; Roizen, M.F. Major Cereal Grain Fibers and Psyllium in Relation to Cardiovascular Health. Nutrients 2013, 5, 1471–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gujral, H.S.; Sharma, P.; Rachna, S. Effect of sand roasting on beta glucan extractability, physicochemical and antioxidant properties of oats. LWT 2011, 44, 2223–2230. [Google Scholar] [CrossRef]
- Ragaee, S.M.; Campbell, G.L.; Scoles, G.J.; McLeod, J.G.; Tyler, R.T. Studies on rye (Secale cereale L.) lines exhibiting a range of extract viscosities. 1. Composition, molecular weight distribution of water extracts, and biochemical characteristics of purified water-extractable arabinoxylan. J. Agric. Food Chem. 2001, 49, 2437–2445. [Google Scholar] [CrossRef]
- Lebiedzinska, A.; Szefer, P. Vitamins B in grain and cereal-grain food, soy-products and seeds. Food Chem. 2006, 95, 116–122. [Google Scholar] [CrossRef]
- Ozawa, H.; Homma, Y.; Arisawa, H.; Fukuuchi, F.; Handa, S. Severe metabolic acidosis and heart failure due to thiamine deficiency. Nutrition 2001, 17, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Falk, J.; Krahnstover, A.; van der Kooij, T.A.W.; Schlensog, M.; Krupinska, K. Tocopherol and tocotrienol accumulation during development of caryopses from barley (Hordeum vulgare L.). Phytochemistry 2004, 65, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Panfili, G.; Fratianni, A.; Di Criscio, T.; Marconi, E. Tocol and beta-glucan levels in barley varieties and in pearling by-products. Food. Chem 2008, 107, 84–91. [Google Scholar] [CrossRef]
- Gangopadhyay, N.; Harrison, S.M.; Brunton, N.P.; Hidalgo-Ruiz, J.L.; Gallagher, E.; Rai, D.K. Brans of the roller-milled barley fractions rich in polyphenols and health-promoting lipophilic molecules. J. Cereal Sci. 2018, 83, 213–221. [Google Scholar] [CrossRef]
- Madhujith, T.; Izydorczyk, M.; Shahidi, F. Antioxidant Properties of Pearled Barley Fractions. J. Agric. Food. Chem. 2006, 54, 3283–3289. [Google Scholar] [CrossRef]
- Gangopadhyay, N.; Rai, D.K.; Brunton, N.P.; Gallagher, E.; Hossain, M.B. Antioxidant-guided isolation and mass spectrometric identification of the major polyphenols in barley (Hordeum vulgare) grain. Food Chem. 2016, 210, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Fisher, N.D.L.; Hughes, M.; Gerhard-Herman, M.; Hollenberg, N.K. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. Am. J. Hypertens. 2003, 21, 2281–2286. [Google Scholar] [CrossRef]
- Grassi, D.; Lippi, C.; Necozione, S.; Desideri, G.; Ferri, C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005, 81, 611–614. [Google Scholar] [CrossRef] [Green Version]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef]
- Sendra, J.M.; Sentandreu, E.; Navarro, J.L. Kinetic model for the antiradical activity of the isolated p-catechol group in flavanone type structures using the free stable radical 2, 2-diphenyl-1-picrylhydrazyl as the antiradical probe. J. Agric. Food Chem. 2007, 55, 5512–5522. [Google Scholar] [CrossRef]
- Kobayashi, A.; Wang, D.; Yamazaki, M.; Tatsumi, N.; Kubota, K. Aroma constituents of tofu (soy bean curd) contributing to its flavor character. J. Jpn. Soc. Food Sci. Technol. 2000, 47, 613–618. [Google Scholar] [CrossRef]
- Yuan, S.H.; Chang, S.K.C. Selected odor compounds in soymilk as affected by chemical composition and lipoxygenases in five soybean materials. J. Agric. Food Chem. 2007, 55, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Shewfelt, R.L.; Lee, K.S.; Kays, S.J. Comparison of odor-active compounds from six distinctly different rice flavor types. J. Agric. Food Chem. 2008, 56, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Cramer, A.C.J.; Mattinson, D.S.; Fellman, J.K.; Baik, B.K. Analysis of volatile compounds from various types of barley cultivars. J. Agric. Food. Chem. 2005, 53, 7526–7531. [Google Scholar] [CrossRef]
- Qin, Z.H.; Pang, X.L.; Chen, D.; Cheng, H.; Hu, X.S.; Wu, J.H. Evaluation of Chinese tea by the electronic nose and gas chromatography-mass spectrometry: Correlation with sensory properties and classification according to grade level. Food Res. Int. 2013, 53, 864–874. [Google Scholar] [CrossRef]
- Takemitsu, H.; Amako, M.; Sako, Y.; Kita, K.; Ozeki, T.; Inui, H.; Kitamura, S. Reducing the undesirable odor of barley by cooking with superheated steam. J. Food Sci. Technol. Mysore 2019, 56, 4732–4741. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendia, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food. Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Ajarayasiri, J.; Chaiseri, S. Comparative study on aroma-active compounds in Thai, black and white glutinous rice varieties. Agric. Nat. Resourc. 2008, 42, 715–722. [Google Scholar]
- Viscidi, K.A.; Dougherty, M.P.; Briggs, J.; Camire, M.E. Complex phenolic compounds reduce lipid oxidation in extruded oat cereals. LWT 2004, 37, 789–796. [Google Scholar] [CrossRef]
- Heiniö, R.L.; Noort, M.W.J.; Katina, K.; Alam, S.A.; Sozer, N.; De Kock, H.L.; Hersleth, M.; Poutanen, K. Sensory characteristics of wholegrain and bran-rich cereal foods–A review. Trends Food Sci. Technol. 2016, 47, 25–38. [Google Scholar] [CrossRef] [Green Version]





| Waxy Barley *1 | Rice Grain Barley *2 | Rolled Barley *2 | |||
|---|---|---|---|---|---|
| PWBP | IBLP | OBLP | |||
| Energy (kcal/100 g) | 367 | 370 | 402 | 333 | 329 |
| Moisture (g/100 g) | 8.8 | 10.5 | 7.4 | 14.0 | 12.7 |
| Protein (g/100 g) | 6.5 | 11.4 | 14.9 | 7.0 | 6.7 |
| Fat (g/100 g) | 1.1 | 4.5 | 9.5 | 2.1 | 1.5 |
| Carbohydrate (g/100 g) | 82.8 | 71.0 | 64.1 | 76.2 | 78.3 |
| Ash (g/100 g) | 0.8 | 2.6 | 4.1 | 0.7 | 0.7 |
| Waxy Barley | Rice Grain Barley *1 | Rolled Barley *1 | |||
|---|---|---|---|---|---|
| PWBP | IBLP | OBLP | |||
| Na (mg/100 g) | 6.29 ± 0.09 a | 12.22 ± 0.23 b | 12.04 ± 0.34 b | 2 | 2 |
| K (mg/100 g) | 62.93 ± 0.86 a | 122.25 ± 2.28 b | 120.43 ± 3.39 b | 170 | 210 |
| Ca (mg/100 g) | 30.09 ± 6.21 | 30.31 ± 0.23 | 59.67 ± 14.30 | 17 | 21 |
| Mg (mg/100 g) | 39.13 ± 0.78 a | 181.17 ± 0.35 b | 353.75 ± 6.15 c | 25 | 40 |
| Fe (mg/100 g) | 4.18 ± 0.04 a | 7.46 ± 0.08 b | 10.52 ± 0.12 c | 1.2 | 1.1 |
| Zn (mg/100 g) | 9.26 ± 0.36 a | 26.76 ± 0.06 b | 50.27 ± 1.06 c | 1.2 | 1.1 |
| Cu (mg/100 g) | 7.84 ± 3.57 | 4.37 ± 0.13 | 7.09 ± 0.12 | 0.37 | 0.22 |
| Mn (mg/100 g) | 10.65 ± 0.21 a | 14.51 ± 0.09 b | 30.16 ± 0.96 c | ― | 0.86 |
| Waxy Barley *1 | Rice Grain Barley *2 | Rolled Barley *2 | |||
|---|---|---|---|---|---|
| PWBP | IBLP | OBLP | |||
| Total fatty acids (g/100 g) | 1.15 | 3.89 | 8.18 | 1.69 | 1.18 |
| Saturated fatty acids (g/100 g) | 0.40 | 0.94 | 1.83 | 0.58 | 0.43 |
| Monounsaturated fatty acids (g/100 g) | 0.08 | 0.69 | 1.53 | 0.20 | 0.13 |
| Polyunsaturated fatty acids (g/100 g) | 0.67 | 2.26 | 4.82 | 0.91 | 0.62 |
| Myristic acid (g/100 g) | ― | ― | 0.02 | 0.008 | 0.006 |
| Pentadecanoic acid (g/100 g) | ― | ― | 0.01 | 0.001 | 0.001 |
| Palmitic acid (g/100 g) | 0.37 | 0.90 | 1.70 | 0.53 | 0.39 |
| Palmitoleic acid (g/100 g) | ― | ― | 0.10 | 0.001 | 0.001 |
| Stearic acid (g/100 g) | 0.03 | 0.04 | 0.06 | 0.025 | 0.022 |
| Oleic acid (g/100 g) | 0.08 | 0.63 | 1.37 | ― | 0.099 |
| Linoleic acid (g/100 g) | 0.64 | 2.12 | 4.40 | 0.86 | 0.59 |
| Alpha-linolenic acid (g/100 g) | 0.03 | 0.14 | 0.42 | 0.054 | 0.033 |
| Arachidic acid (g/100 g) | ― | ― | 0.02 | 0.002 | 0.002 |
| Eicosenoic acid (g/100 g) | ― | 0.03 | 0.09 | 0.010 | 0.006 |
| Behenic acid (g/100 g) | ― | ― | 0.02 | 0.007 | 0.002 |
| Docosenoic acid (g/100 g) | ― | 0.03 | 0.05 | 0.017 | 0.011 |
| Tetracosenoic acid (g/100 g) | ― | ― | 0.01 | 0.000 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuichi, T.; Abe, D.; Uchikawa, T.; Nagasaki, T.; Kanou, M.; Kasuga, J.; Matsumoto, S.; Tsurunaga, Y. Comparison of Nutritional Composition and Antioxidant Properties of Pulverized and Unutilized Portions of Waxy Barley. Foods 2023, 12, 2639. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12142639
Furuichi T, Abe D, Uchikawa T, Nagasaki T, Kanou M, Kasuga J, Matsumoto S, Tsurunaga Y. Comparison of Nutritional Composition and Antioxidant Properties of Pulverized and Unutilized Portions of Waxy Barley. Foods. 2023; 12(14):2639. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12142639
Chicago/Turabian StyleFuruichi, Tsugumi, Daigo Abe, Takuya Uchikawa, Toshihiro Nagasaki, Mina Kanou, Junko Kasuga, Shingo Matsumoto, and Yoko Tsurunaga. 2023. "Comparison of Nutritional Composition and Antioxidant Properties of Pulverized and Unutilized Portions of Waxy Barley" Foods 12, no. 14: 2639. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12142639