Characterization of Aroma Active Compound Production during Kombucha Fermentation: Towards the Control of Sensory Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
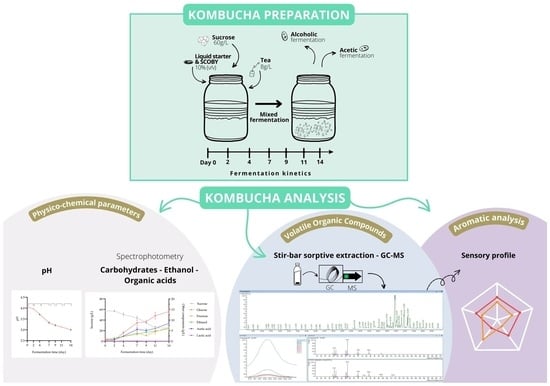
2.2. Kombucha Preparation
2.3. Dynamic Analysis of the Physico-Chemical Parameters of the Drink
2.4. Chromatographic Analysis of the Development of Volatile Organic Compounds
2.5. Raw Data Analysis
3. Results and Discussions
3.1. Physico-Chemical Characterization of Kombucha during Fermentation
3.1.1. Evolution of pH during Fermentation
3.1.2. Carbohydrate, Alcohol, and Acid Concentration Kinetics during Kombucha Fermentation
3.2. Study of the Development Kinetics of Volatile Organic Compounds
3.2.1. Chemical Analysis of Major VOCs and Metabolic Pathways Involved in Fermentation
3.2.2. Aromatic Characterization of Kombucha and Establishment of Sensory Profiles
4. Conclusions
5. Prospects of this Research Study
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gou, M.; Bi, J.; Chen, Q.; Wu, X.; Fauconnier, M.L.; Qiao, Y. Advances and Perspectives in Fruits and Vegetables Flavor Based on Molecular Sensory Science. Food Rev. Int. 2021, 1–14. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for Future Food Systems. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef] [PubMed]
- Lonǎr, E.; Djurić, M.; Malbaša, R.; Kolarov, L.J.; Klašnja, M. Influence of Working Conditions Upon Kombucha Conducted Fermentation of Black Tea. Food Bioprod. Process. 2006, 84, 186–192. [Google Scholar] [CrossRef]
- Dufresne, C.; Farnworth, E. Tea, Kombucha, and Health: A Review. Food Res. Int. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Teoh, A.L.; Heard, G.; Cox, J. Yeast Ecology of Kombucha Fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Kombucha: Biochemical and Microbiological Impacts on the Chemical and Flavor Profile. Food Chem. Adv. 2022, 1, 100025. [Google Scholar] [CrossRef]
- de Oliveira, Í.A.C.L.; Rolim, V.A.D.O.; Gaspar, R.P.L.; Rossini, D.Q.; de Souza, R.; Bogsan, C.S.B. The Technological Perspectives of Kombucha and Its Implications for Production. Fermentation 2022, 8, 185. [Google Scholar] [CrossRef]
- Sievers, M.; Lanini, C.; Weber, A.; Schuler-Schmid, U.; Teuber, M. Microbiology and Fermentation Balance in a Kombucha Beverage Obtained from a Tea Fungus Fermentation. Syst. Appl. Microbiol. 1995, 18, 590–594. [Google Scholar] [CrossRef]
- McNeil, B.; Harvey, L.M.; Rowan, N.J.; Giavasis, I. Fermentation Monitoring and Control of Microbial Cultures for Food Ingredient Manufacture. Microb. Prod. Food Ingred. Enzym. Nutraceuticals 2013, 125–143. [Google Scholar] [CrossRef]
- Markov, S.L.; Malbaša, R.V.; Hauk, M.J.; Cvetković, D.D. Investigation of Tea Fungus Microbe Assotiations: I: The Yeasts. Acta Period. Technol. 2001, 133–138. [Google Scholar]
- Règlement (UE) n o 1169/2011 du Parlement Européen et du Conseil du 25 Octobre 2011 Concernant L’information des Consommateurs sur les Denrées Alimentaires, Modifiant les Règlements (CE) n o 1924/2006 et (CE) n o 1925/2006 du Parlement Européen et du Conseil et Abrogeant la Directive 87/250/CEE de la Commission, la Directive 90/496/CEE du Conseil, la Directive 1999/10/CE de la Commission, la Directive 2000/13/CE du Parlement Européen et du Conseil, les Directives 2002/67/CE et 2008/5/CE de la Commission et le Règlement (CE) n o 608/2004 de la Commission Texte Présentant de L’intérêt pour l’EEE. 2011; Volume 304.
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Health, Wellness, and Safety Aspects of the Consumption of Kombucha. J. Chem. 2015, 2015, 591869. [Google Scholar] [CrossRef] [Green Version]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [Green Version]
- Martínez Leal, J.; Valenzuela Suárez, L.; Jayabalan, R.; Huerta Oros, J.; Escalante-Aburto, A. A Review on Health Benefits of Kombucha Nutritional Compounds and Metabolites. CyTA—J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea—Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha Beverage from Green, Black and Rooibos Teas: A Comparative Study Looking at Microbiology, Chemistry and Antioxidant Activity. Nutrients 2019, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Jayabalan, R.; Malini, K.; Sathishkumar, M.; Swaminathan, K.; Yun, S.E. Biochemical Characteristics of Tea Fungus Produced during Kombucha Fermentation. Food Sci. Biotechnol. 2010, 19, 843–847. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in Content of Organic Acids and Tea Polyphenols during Kombucha Tea Fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the Fermented Tea: Microbiology, Composition, and Claimed Health Effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef]
- Talebi, M.; Frink, L.A.; Patil, R.A.; Armstrong, D.W. Examination of the Varied and Changing Ethanol Content of Commercial Kombucha Products. Food Anal. Methods 2017, 10, 4062–4067. [Google Scholar] [CrossRef]
- Harrison, K.; Curtin, C. Microbial Composition of SCOBY Starter Cultures Used by Commercial Kombucha Brewers in North America. Microorganisms 2021, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Karabiyikli, S. Importance of Acetic Acid Bacteria in Food Industry. Food Control. 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Paslier, D.L.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Behera, S.S.; Ray, R.C.; Das, U.; Panda, S.K.; Saranraj, P. Microorganisms in Fermentation. Learn. Mater. Biosci. 2019, 1–39. [Google Scholar] [CrossRef]
- Dutta, H.; Paul, S.K. Kombucha Drink: Production, Quality, and Safety Aspects. Prod. Manag. Beverages 2019, 259–288. [Google Scholar] [CrossRef]
- LiangLiang, G.; XiaoYan, H.; Yue, L.; QingFu, C. Production Technology Optimization on Fermented Tea of Golden Buckwheat. Guizhou Agric. Sci. 2014, 42, 169–174. [Google Scholar]
- Pederson, C.S. Microbiology of Food Fermentations; AVI Publishing Co. Inc.: Westport, CO, USA, 1979; p. 384. [Google Scholar]
- Filippis, F.D.; Troise, A.D.; Vitaglione, P.; Ercolini, D. Different Temperatures Select Distinctive Acetic Acid Bacteria Species and Promotes Organic Acids Production during Kombucha Tea Fermentation. Food Microbiol. 2018, 73, 11–16. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha Tea Fermentation: Microbial and Biochemical Dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Reva, O.N.; Zaets, I.E.; Ovcharenko, L.P.; Kukharenko, O.E.; Shpylova, S.P.; Podolich, O.V.; de Vera, J.P.; Kozyrovska, N.O. Metabarcoding of the Kombucha Microbial Community Grown in Different Microenvironments. AMB Express 2015, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of Potentially Probiotic Lactic Acid Bacteria on the Physicochemical Composition and Acceptance of Fermented Cereal Beverages. J. Funct. Foods 2015, 15, 106–115. [Google Scholar] [CrossRef]
- Laureys, D.; Britton, S.J.; De Clippeleer, J. Kombucha Tea Fermentation: A Review. J. Am. Soc. Brew. Chem. 2020, 78, 165–174. [Google Scholar] [CrossRef]
- Zhen-jun Zhao, Y.S.; Wu, H.; Zhou, C.; Xian-chun Hu, J.Z. Flavour Chemical Dynamics during Fermentation of Kombucha Tea. Emir. J. Food Agric. 2018, 732–741. [Google Scholar] [CrossRef]
- Chen, C.; Liu, B.Y. Changes in Major Components of Tea Fungus Metabolites during Prolonged Fermentation. J. Appl. Microbiol. 2000, 89, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Van Mullem, J.; Dias, D.R.; Schwan, R.F. The Chemistry and Sensory Characteristics of New Herbal Tea-Based Kombuchas. J. Food Sci. 2021, 86, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M. Analysis of Volatiles in Food Products. Separations 2021, 8, 157. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K.; David, F.; Sandra, P. Recent Developments of Stir Bar Sorptive Extraction for Food Applications: Extension to Polar Solutes. J. Agric. Food Chem. 2018, 66, 7249–7255. [Google Scholar] [CrossRef]
- Staff, D.A.; Register, O. of the F. Code of Federal Regulations: Title 21: Food and Drugs; Office of the Federal Register: Washington, DC, USA, 2005. [Google Scholar]
- David, F.; Sandra, P. Stir Bar Sorptive Extraction for Trace Analysis. J. Chromatography. A 2007, 1152, 54–69. [Google Scholar] [CrossRef]
- Margoum, C.; Guillemain, C.; Yang, X.; Coquery, M.; Margoum, C.; Guillemain, C.; Yang, X. Stir Bar Sorptive Extraction Coupled to Liquid Chromatography-Tandem Mass Spectrometry for the Determination of Pesticides in Water Samples: Method Validation and Measurement Uncertainty Title: 1 Stir Bar Sorptive Extraction Coupled to Liquid Chromatography-Tandem Mass Spectrometry for the 2 Determination of Pesticides in Water Samples: Method Validation and Measurement Uncertainty. Talanta 2013, 116, 1–7. [Google Scholar]
- Vedenin, A.; Suvorkin, V.; Kachur, E. Stir Bar Sorptive Extraction-Thermal Desorption-Capillary GC-MS Applied for Analysis of Amphetamine Derivatives in Biological Fluids. GERSTEL AppNote 2014, 7. [Google Scholar]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of Substrate Composition and Inoculum on the Fermentation Kinetics and Flavour Compound Profiles of Potentially Non-Dairy Probiotic Formulations. LWT—Food Sci. Technol. 2014, 55, 240–247. [Google Scholar] [CrossRef]
- Parker, J.K.; Elmore, S.; Methven, L. Flavour Development, Analysis and Perception in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-1-78242-111-5. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A Chemical Data Resource on the Internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]
- The Good Scents Company—Flavor, Fragrance, Food and Cosmetics Ingredients Information. Available online: http://www.thegoodscentscompany.com/ (accessed on 10 January 2023).
- Gou, M.; Chen, Q.; Wu, X.; Liu, G.; Fauconnier, M.-L.; Bi, J. Novel Insight into the Evolution of Volatile Compounds during Dynamic Freeze-Drying of Ziziphus Jujuba Cv. Huizao Based on GC–MS Combined with Multivariate Data Analysis. Food Chem. 2023, 410, 135368. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of Fermentation Conditions on the Production of Bioactive Compounds with Anticancer, Anti-Inflammatory and Antioxidant Properties in Kombucha Tea Extracts. Process. Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Zailani, N.S.; Adnan, A. Substrates and metabolic pathways in symbiotic culture of bacteria and yeast (scoby) fermentation: A mini review. J. Teknol. 2022, 84, 155–165. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid Contents and the Effect of Fermentation Condition of Kombucha Tea Beverages on Physicochemical, Microbiological and Sensory Properties. Cyta-J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial Cellulose—The Natural Power to Heal Wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef]
- Balentine, D.A.; Wiseman, S.A.; Bouwens, L.C.M. The Chemistry of Tea Flavonoids. Crit. Rev. Food Sci. Nutr. 1997, 37, 693–704. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, D.; Chen, H.; Qian, L.; Xu, P. Fatty Acid Composition and Antioxidant Activity of Tea (Camellia sinensis L.) Seed Oil Extracted by Optimized Supercritical Carbon Dioxide. Int. J. Mol. Sci. 2011, 12, 7708–7719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Yang, M.; Fu, J. Identification and Characterization of Two Sesquiterpene Synthase Genes Involved in Volatile-Mediated Defense in Tea Plant (Camellia sinensis). Plant Physiol. Biochem. 2020, 155, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.; Yang, Z. Characterization of Terpene Synthase from Tea Green Leafhopper Being Involved in Formation of Geraniol in Tea (Camellia sinensis) Leaves and Potential Effect of Geraniol on Insect-Derived Endobacteria. Biomolecules 2019, 9, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of Innovation in Health and Disease. Chem. -Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, C.; Scott, T.R. Food Flavours; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997. [Google Scholar] [CrossRef]
- Chan, M.; Sy, H.; Finley, J.; Robertson, J.; Brown, P.N. Determination of Ethanol Content in Kombucha Using Headspace Gas Chromatography with Mass Spectrometry Detection: Single-Laboratory Validation. J. AOAC Int. 2021, 104, 122–128. [Google Scholar] [CrossRef]
- Bamforth, C.W.; Cook, D.J. Food, Fermentation, and Micro-Organisms, 2nd ed.; 2019; pp. 1–245. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. MVOC 2.0: A Database of Microbial Volatiles. Nucleic Acids Res. 2018, 46, D1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guittin, C.; Maçna, F.; Sanchez, I.; Barreau, A.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. The Impact of Must Nutrients and Yeast Strain on the Aromatic Quality of Wines for Cognac Distillation. Fermentation 2022, 8, 51. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Jin, Y.; Liu, J.; Deng, L.; Wang, F. Recent Advances in Multiple Strategies for the Synthesis of Terpenes by Engineered Yeast. Fermentation 2022, 8, 615. [Google Scholar] [CrossRef]
- Ferremi Leali, N.; Binati, R.L.; Martelli, F.; Gatto, V.; Luzzini, G.; Salini, A.; Slaghenaufi, D.; Fusco, S.; Ugliano, M.; Torriani, S.; et al. Reconstruction of Simplified Microbial Consortia to Modulate Sensory Quality of Kombucha Tea. Foods 2022, 11, 3045. [Google Scholar] [CrossRef]
- Saberi, S.; Cliff, M.A.; van Vuuren, H.J.J. Impact of Mixed S. Cerevisiae Strains on the Production of Volatiles and Estimated Sensory Profiles of Chardonnay Wines. Food Res. Int. 2012, 48, 725–735. [Google Scholar] [CrossRef]
- Abaci, N.; Senol Deniz, F.S.; Orhan, I.E. Kombucha—An Ancient Fermented Beverage with Desired Bioactivities: A Narrowed Review. Food Chem. X 2022, 14, 100302. [Google Scholar] [CrossRef]
- Bullerman, L.B. SPOILAGE|Fungi in Food—An Overview. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 5511–5522. ISBN 978-0-12-227055-0. [Google Scholar]
- Ignat, M.V.; Salanță, L.C.; Pop, O.L.; Pop, C.R.; Tofană, M.; Mudura, E.; Coldea, T.E.; Borșa, A.; Pasqualone, A. Current Functionality and Potential Improvements of Non-Alcoholic Fermented Cereal Beverages. Foods 2020, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-Based Fermented Foods and Beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Aly, M.M.A.; Hassan, A.A.; El-Hadidie, S.T. Production of Cereal-Based Probiotic Beverages. World Appl. Sci. J. 2012, 19, 1367–1380. [Google Scholar]








| Raw Matrix | Kombucha Fermentation Stages | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Compound | CAS Number | RI 2 | RI lit 3 | Black Tea | Green Tea | D0 | D2 | D4 | D7 | D9 | D11 | D14 |
| Carboxylic acid | (2E)-3,7-dimethylocta-2,6-dienoic acid | 4698-08-2 | 2326 | 2329 | nd | nd | 5.02 ± 0.26 | 4.68 ± 0.84 | 4.86 ± 0.34 | 5.24 ± 0.78 | 6.40 ± 0.91 | 7.08 ± 0.81 | 5.93 ± 1.47 |
| (9E,12E)-octadeca-9,12-dienoic acid | 506-21-8 | 3171 | 3168 | nd | nd | nd | nd | 7.15 ± 0.50 | nd | nd | nd | nd | |
| (E)-hexadec-9-enoic acid | 10030-73-6 | 2570 | 2579 | 226.30 | 129.69 | nd | nd | 2.36 ± 0.53 | nd | 7.81 ± 4.21 | 10.96 ± 1.02 | nd | |
| (E)-oct-2-enoic acid | 1871-67-6 | 2084 | 2082 | nd | nd | 1.84 ± 0.66 | 1.26 ± 0.29 | nd | nd | nd | nd | nd | |
| (Z)-heptadec-10-enoic acid | 29743-97-3 | 3036 | 3030 | 30.22 | nd | 8.99 ± 0.15 | 2.09 ± 0.66 | 19.46 ± 6.74 | 7.55 ± 1.18 | 4.45 ± 3.00 | 7.35 ± 0.46 | 2.18 ± 0.63 | |
| (Z)-hexadec-7-enoic acid | 2416-19-5 | 2920 | 2924 | nd | nd | nd | nd | 14.03 ± 16.43 | nd | 33.57 ± 8.63 | nd | nd | |
| (Z)-hexadec-9-enoic acid | 373-49-9 | 2932 | 2944 | nd | nd | 21.21 ± 0.84 | 45.90 ± 3.93 | 123.34 ± 2.55 | 58.62 ± 5.45 | 41.50 ± 13.58 | 57.22 ± 11.48 | 17.34 ± 10.54 | |
| (Z)-nonadec-10-enoic acid | 73033-09-7 | 3258 | 3256 | nd | nd | nd | 29.08 ± 6.69 | nd | 28.66 ± 2.64 | nd | nd | nd | |
| (Z)-octadec-11-enoic acid | 506-17-2 | 3132 | 3135 | nd | nd | nd | nd | nd | 37.21 ± 12.61 | 26.58 ± 6.89 | 33.99 ± 7.19 | 9.91 ± 3.30 | |
| (Z)-octadec-9-enoic acid | 112-80-1 | 3133 | 3142 | nd | 198.03 | nd | nd | 71.75 ± 98.09 | nd | nd | nd | nd | |
| (Z)-tetradec-9-enoic acid | 544-64-9 | 3168 | 3173 | 19.90 | nd | nd | 1.82 ± 0.21 | 7.57 ± 6.16 | 2.59 ± 1.01 | 2.62 ± 1.34 | 2.92 ± 1.98 | nd | |
| 2-methylpropanoic acid | 79-31-2 | 1565 | 1563 | nd | nd | nd | nd | 2.71 ± 0.04 | 3.03 ± 0.30 | 2.93 ± 1.08 | 5.84 ± 0.40 | 8.13 ± 1.48 | |
| 3-methylbutanoic acid | 503-74-2 | 1665 | 1664 | nd | nd | 5.81 ± 0.47 | 7.66 ± 1.41 | 7.74 ± 0.21 | 13.86 ± 0.88 | 17.54 ± 4.19 | 31.95 ± 3.24 | 40.74 ± 10.26 | |
| acetic acid | 64-19-7 | 1450 | 1450 | nd | nd | nd | nd | nd | nd | nd | nd | 15.17 ± 9.76 | |
| butanoic acid | 107-92-6 | 1623 | 1628 | nd | nd | nd | nd | 4.15 ± 1.73 | 1.86 ± 0.23 | nd | nd | nd | |
| dec-9-enoic acid | 14436-32-9 | 2300 | 2305 | nd | nd | nd | nd | nd | nd | nd | nd | 0.99 ± 0.24 | |
| decanoic acid | 334-48-5 | 2264 | 2267 | 21.83 | 17.10 | 13.13 ± 1.01 | 3.48 ± 0.27 | 30.58 ± 0.74 | 62.49 ± 1.74 | 71.98 ± 4.28 | 94.60 ± 3.52 | 89.08 ± 7.94 | |
| dodecanoic acid | 143-07-7 | 2474 | 2471 | 11.96 | 7.04 | 3.03 ± 0.67 | 1.84 ± 0.48 | 11.33 ± 3.14 | 4.83 ± 0.60 | 3.71 ± 1.03 | 4.72 ± 0.84 | 1.98 ± 0.61 | |
| heptadecanoic acid | 506-12-7 | 3027 | 3027 | 19.93 | nd | nd | 4.74 ± 0.96 | 14.57 ± 4.82 | 6.35 ± 1.17 | 5.02 ± 0.35 | 5.46 ± 1.72 | 2.15 ± 0.28 | |
| heptanoic acid | 111-14-8 | 1944 | 1946 | 2.20 | nd | 7.97 ± 0.89 | 6.44 ± 1.53 | 6.65 ± 2.39 | 7.24 ± 1.91 | 9.32 ± 1.77 | 9.64 ± 1.36 | 9.07 ± 1.79 | |
| hexadecanoic acid | 57-10-3 | 2898 | 2890 | 568.52 | 427.35 | 80.04 ± 0.21 | 97.89 ± 8.45 | 234.64 ± 41.41 | 117.93 ± 11.21 | 100.84 ± 28.58 | 98.64 ± 16.32 | 47.75 ± 12.37 | |
| hexanoic acid | 142-62-1 | 1838 | 1838 | nd | nd | 21.23 ± 0.92 | 29.42 ± 6.91 | 43.10 ± 3.45 | 56.00 ± 9.57 | 44.22 ± 6.62 | 60.84 ± 10.69 | 51.37 ± 9.61 | |
| nonanoic acid | 112-05-0 | 2171 | 2174 | 11.07 | nd | 16.73 ± 0.07 | 3.54 ± 0.79 | 14.45 ± 0.22 | 18.17 ± 0.18 | 13.72 ± 0.61 | 14.84 ± 1.65 | 13.91 ± 1.11 | |
| octadecanoic acid | 57-11-4 | 3107 | 3104 | 11.36 | nd | 5.01 ± 0.60 | 8.66 ± 3.73 | 37.79 ± 6.50 | 12.45 ± 1.02 | 32.22 ± 1.67 | 22.59 ± 2.55 | 13.44 ± 8.88 | |
| octanoic acid | 124-07-2 | 2052 | 2050 | 5.44 | 6.07 | 74.71 ± 0.69 | 164.58 ± 15.00 | 284.03 ± 15.47 | 313.86 ± 10.05 | 321.71 ± 17.22 | 339.21 ± 23.23 | 303.54 ± 9.94 | |
| pentadecanoic acid | 1002-84-2 | 2741 | 2745 | 150.78 | 102.42 | 16.04 ± 0.65 | 24.28 ± 3.39 | 74.71 ± 23.23 | 36.10 ± 5.89 | 23.62 ± 8.84 | 30.41 ± 7.96 | 10.27 ± 2.85 | |
| tetradecanoic acid | 544-63-8 | 2656 | 2660 | 134.58 | 107.04 | 18.04 ± 0.08 | 21.12 ± 1.67 | 65.28 ± 17.97 | 31.26 ± 3.45 | 22.05 ± 11.59 | 22.07 ± 3.98 | 8.83 ± 2.93 | |
| tridecanoic acid | 638-53-9 | 2580 | 2573 | 8.57 | nd | nd | nd | 4.12 ± 1.12 | 1.38 ± 0.04 | 1.04 ± 0.45 | 1.22 ± 0.07 | nd | |
| Total | 1222.65 | 994.75 | 298.2 | 448.38 | 1086.38 | 816.65 | 781.66 | 861.16 | 653.95 | ||||
| Alcohol | geraniol | 106-24-1 | 1840 | 1836 | 44.04 | nd | 18.03 ± 0.79 | 16.34 ± 0.71 | 15.32 ± 0.80 | nd | 13.88 ± 0.91 | 12.78 ± 1.80 | 12.09 ± 1.74 |
| nerol | 106-25-2 | 1793 | 1794 | 3.28 | nd | 1.98 ± 0.13 | 2.14 ± 0.09 | 2.11 ± 0.18 | 2.70 ± 0.07 | 3.08 ± 0.19 | 3.40 ± 0.19 | 3.16 ± 0.11 | |
| linaloolpyran oxide C | 10448-31-4 | 1755 | 1741 | 9.58 | nd | 1.91 ± 0.11 | 3.10 ± 0.55 | 1.87 ± 0.21 | 1.53 ± 0.12 | 1.28 ± 0.25 | 1.67 ± 0.29 | 1.32 ± 0.07 | |
| hotrienol | 29957-43-5 | 1608 | 1613 | nd | 2.09 | nd | 1.94 ± 0.52 | 1.41 ± 0.04 | 1.19 ± 0.32 | 1.22 ± 0.27 | 1.83 ± 0.07 | 2.09 ± 0.41 | |
| (Z)-hex-3-en-1-ol | 928-96-1 | 1383 | 1390 | nd | nd | 1.11 ± 0.40 | 2.34 ± 0.81 | 2.10 ± 0.06 | 1.83 ± 0.06 | 1.77 ± 0.33 | 2.13 ± 0.33 | 1.79 ± 0.18 | |
| 1-methoxypropan-2-ol | 107-98-2 | 1152 | 1160 | nd | nd | 47.78 ± 1.10 | 47.04 ± 18.20 | 9.54 ± 5.04 | 9.64 ± 1.81 | 17.69 ± 5.07 | 17.89 ± 3.73 | nd | |
| 8-camphenemethanol | 2226-05-3 | 1688 | 1682 | nd | nd | 1.11 ± 0.15 | 0.92 ± 0.07 | nd | nd | nd | nd | nd | |
| linalool oxide | 60047-17-8 | 1440 | 1442 | nd | nd | 10.87 ± 1.01 | 10.03 ± 1.01 | nd | nd | nd | nd | nd | |
| 2,6-dimethylcyclohexan-1-ol | 5337-72-4 | 1112 | 1114 | nd | nd | 5.53 ± 0.70 | 4.27 ± 0.43 | 2.83 ± 0.06 | 2.08 ± 0.01 | 1.38 ± 0.42 | 1.75 ± 0.10 | 1.38 ± 0.00 | |
| (-)-α-terpineol | 10482-56-1 | 1690 | 1690 | nd | nd | nd | 13.22 ± 0.76 | 11.34 ± 1.32 | 13.22 ± 0.35 | 13.16 ± 1.05 | 18.11 ± 1.45 | 16.52 ± 1.24 | |
| trans-furan linalool oxide | 34995-77-2 | 1468 | 1460 | 109.31 | nd | 15.95 ± 0.35 | 15.90 ± 1.93 | 10.64 ± 0.30 | 7.97 ± 0.27 | 5.95 ± 1.08 | 6.95 ± 1.02 | 5.28 ± 0.37 | |
| 2-ethylhexan-1-ol | 104-76-7 | 1489 | 1490 | 10.06 | 6.86 | 3.95 ± 0.10 | 8.66 ± 0.60 | 9.18 ± 0.41 | 11.76 ± 0.42 | 7.75 ± 0.39 | 7.83 ± 1.38 | 7.94 ± 0.28 | |
| 2-phenylethanol | 60-12-8 | 1897 | 1890 | 1.97 | nd | 35.83 ± 0.53 | 77.78 ± 13.55 | 135.58 ± 3.04 | 225.04 ± 5.48 | 242.05 ± 20.25 | 288.59 ± 26.39 | 273.15 ± 2.16 | |
| linalool | 78-70-6 | 1546 | 1545 | 222.28 | 5.44 | 72.18 ± 2.10 | 45.82 ± 2.63 | 33.83 ± 1.99 | 38.39 ± 3.18 | 43.87 ± 4.91 | 53.14 ± 6.28 | 48.69 ± 2.55 | |
| 3-methylbutan-1-ol | 123-51-3 | 1216 | 1218 | nd | nd | 22.30 ± 3.55 | 27.37 ± 8.73 | 41.89 ± 0.86 | 66.49 ± 11.82 | 61.35 ± 29.29 | 115.60 ± 28.99 | 113.39 ± 8.02 | |
| 4,8-dimethylnona-1,7-dien-4-ol | 17920-92-2 | 1182 | 1191 | nd | nd | nd | 1.27 ± 0.17 | nd | nd | nd | nd | nd | |
| 4-carvomenthenol | 562-74-3 | 1597 | 1594 | 1.34 | nd | 1.29 ± 0.42 | 0.88 ± 0.12 | nd | nd | nd | nd | nd | |
| butan-1-ol | 71-36-3 | 1157 | 1157 | nd | 1.01 | nd | 3.29 ± 2.61 | 3.18 ± 1.57 | 2.96 ± 1.24 | nd | 3.12 ± 2.19 | 2.77 ± 0.16 | |
| heptan-3-ol | 589-82-2 | 1317 | 1306 | nd | nd | nd | 1.50 ± 0.39 | nd | nd | nd | nd | nd | |
| hexan-1-ol | 111-27-3 | 1354 | 1356 | 7.27 | nd | 10.88 ± 0.53 | 5.23 ± 1.02 | 1.75 ± 0.23 | 1.27 ± 0.14 | nd | nd | nd | |
| nonan-1-ol | 143-08-8 | 1657 | 1666 | 1.12 | nd | 1.89 ± 0.17 | 2.38 ± 0.13 | 3.14 ± 0.03 | 1.62 ± 0.21 | nd | nd | nd | |
| oct-1-en-3-ol | 3391-86-4 | 1450 | 1456 | 3.80 | nd | 1.91 ± 0.09 | 1.00 ± 0.24 | nd | nd | nd | nd | nd | |
| octan-1-ol | 111-87-5 | 1556 | 1565 | nd | nd | 5.44 ± 0.77 | 2.73 ± 0.62 | 4.30 ± 0.46 | 3.33 ± 0.31 | 1.76 ± 0.19 | nd | nd | |
| Total | 414.05 | 15.39 | 260.25 | 294.48 | 290.03 | 390.63 | 415.71 | 528.83 | 484.51 | ||||
| Aldehyde | β-cyclocitral | 432-25-7 | 1613 | 1611 | 13.34 | 30.77 | 5.63 ± 0.56 | 1.96 ± 0.11 | nd | nd | nd | nd | nd |
| 2-phenylacetaldehyde | 122-78-1 | 1635 | 1632 | nd | nd | nd | nd | nd | nd | nd | nd | 1.35 ± 0.00 | |
| 3-methylbenzaldehyde | 620-23-5 | 1638 | 1635 | nd | nd | nd | nd | 3.19 ± 0.04 | nd | nd | nd | nd | |
| benzaldehyde | 100-52-7 | 1611 | 1617 | 10.54 | nd | 34.41 ± 1.19 | nd | nd | 1.78 ± 0.05 | 1.02 ± 0.05 | nd | nd | |
| decanal | 112-31-2 | 1499 | 1498 | 10.80 | nd | nd | 2.20 ± 0.36 | 2.65 ± 0.34 | 2.22 ± 0.2 | 1.61 ± 0.21 | nd | 1.34 ± 0.05 | |
| hexanal | 66-25-1 | 1099 | 1094 | 10.02 | 22.54 | 9.07 ± 0.69 | 6.32 ± 0.44 | 4.73 ± 0.43 | 2.96 ± 1.75 | 1.93 ± 0.40 | 2.68 ± 0.42 | nd | |
| nonanal | 124-19-6 | 1395 | 1390 | 34.69 | nd | nd | 7.06 ± 1.00 | 7.07 ± 0.08 | 6.57 ± 1.11 | 4.94 ± 0.43 | 4.86 ± 1.49 | 4.13 ± 0.39 | |
| Total | 79.40 | 53.32 | 49.12 | 14.71 | 17.63 | 13.54 | 9.51 | 7.53 | 6.37 | ||||
| Benzene | styrene | 100-42-5 | 1258 | 1259 | 17.44 | 62.63 | nd | 1.60 ± 0.98 | nd | nd | nd | nd | nd |
| Total | 17.44 | 62.63 | nd | 1.60 | nd | nd | nd | nd | nd | ||||
| Ketone | cis-dihydrocarvone | 3792-53-8 | 1600 | 1602 | nd | nd | nd | 2.70 ± 0.19 | nd | nd | nd | nd | nd |
| (3E)-6-methylhepta-3,5-dien-2-one | 1604-28-0 | 1588 | 1582 | nd | nd | 1.81 ± 0.32 | 1.14 ± 0.30 | nd | nd | nd | nd | nd | |
| (3E,5E)-octa-3,5-dien-2-one | 30086-02-3 | 1566 | 1565 | nd | nd | 5.08 ± 0.06 | 14.85 ± 1.51 | nd | nd | nd | nd | nd | |
| dihydroactinidiolide | 17092-92-1 | 2291 | 2291 | 7.90 | 96.50 | 7.95 ± 0.16 | 7.98 ± 0.97 | 6.98 ± 0.37 | 5.80 ± 0.42 | 5.37 ± 0.90 | 6.35 ± 0.70 | 5.90 ± 0.31 | |
| dehydro-β-ionone | 1203-08-3 | 1927 | 1932 | nd | nd | nd | nd | nd | 1.06 ± 0.07 | nd | 1.34 ± 0.19 | 1.30 ± 0.24 | |
| β-ionone | 14901-07-6 | 1964 | 1967 | 6.15 | 23.29 | 2.77 ± 0.17 | 1.63 ± 0.06 | nd | nd | nd | nd | nd | |
| 1-(5-methylthiophen-2-yl)ethanone | 13679-74-8 | 1185 | 1197 | 0.89 | 6.45 | nd | nd | nd | nd | 2.13± | 1.27 ± 0.25 | 1.24 ± 0.12 | |
| β-ionone epoxyde | 23267-57-4 | 1963 | 1968 | 6.56 | 61.74 | 4.26 ± 0.40 | 3.52 ± 0.16 | 2.89 ± 0.04 | 2.06 ± 0.12 | 1.44 ± 0.15 | nd | nd | |
| 5-methylheptan-2-one | 18217-12-4 | 1145 | 1152 | 4.58 | 91.06 | nd | nd | nd | nd | nd | nd | 2.26 ± 0.34 | |
| 6-methylhept-5-en-2-one | 110-93-0 | 1337 | 1340 | 12.82 | 3.09 | 7.01 ± 0.29 | 3.94 ± 0.75 | 2.04 ± 0.14 | 2.19 ± 0.47 | 2.08 ± 0.05 | 2.17 ± 0.20 | 1.38 ± 0.31 | |
| Total | 38.90 | 282.12 | 28.87 | 35.77 | 11.91 | 11.12 | 11.69 | 10.41 | 11.33 | ||||
| (1-ethynylcyclohexyl) carbamate | 126-52-3 | 1316 | 1313 | nd | nd | nd | nd | 3.37 ± 0.03 | nd | nd | nd | nd | |
| 2-(1H-indol-3-yl)ethyl acetate | 13137-14-9 | 1863 | 1865 | nd | nd | nd | nd | 10.21 ± 4.16 | 20.53 ± 3.19 | 31.63 ± 6.01 | 25.18 ± 1.11 | 20.59 ± 1.53 | |
| 2-phenylethyl acetate | 103-45-7 | 1806 | 1808 | nd | nd | 4.63 ± 0.53 | 3.93 ± 0.56 | 7.12 ± 0.53 | 12.93 ± 1.03 | 19.63 ± 3.23 | 28.51 ± 2.47 | 40.66 ± 4.15 | |
| Ester | ethyl 2-phenylacetate | 101-97-3 | 1777 | 1763 | nd | nd | nd | nd | nd | nd | 1.25 ± 0.13 | 1.55 ± 0.07 | 1.86 ± 0.06 |
| ethyl hexanoate | 123-66-0 | 1239 | 1241 | nd | nd | nd | nd | nd | 2.36 ± 0.38 | 3.31 ± 0.76 | 3.99 ± 1.13 | 5.87 ± 1.53 | |
| methyl 2-hydroxybenzoate | 119-36-8 | 1764 | 1766 | 17.03 | nd | 17.61 ± 0.59 | 10.05 ± 0.86 | nd | nd | nd | nd | nd | |
| methyl hexanoate | 106-70-7 | 1190 | 1184 | nd | nd | 1.45 ± 0.67 | 3.60 ± 3.41 | 2.50 ± 0.73 | nd | 1.46 ± 0.28 | 2.73 ± 0.52 | 2.73 ± 0.24 | |
| Total | 17.03 | 0.00 | 23.70 | 16.38 | 15.47 | 35.81 | 46.74 | 61.44 | 71.71 | ||||
| Phenol | 4-ethyl-2-methoxyphenol | 2785-89-9 | 2016 | 2020 | nd | nd | 7.86 ± 0.39 | 11.82 ± 1.11 | 29.02 ± 1.53 | 30.88± | 30.85± | 32.44 ± 2.46 | 29.59 ± 1.71 |
| 4-ethylphenol | 123-07-9 | 2161 | 2164 | nd | nd | 7.99 ± 0.08 | 10.54 ± 1.18 | 28.53 ± 0.10 | 29.04± | 27.10± | 28.74 ± 3.23 | 24.46 ± 2.22 | |
| Total | nd | nd | 16.02 | 22.36 | 57.55 | 59.92 | 57.95 | 61.18 | 54.06 | ||||
| α-farnesene | 502-61-4 | 1687 | 1695 | nd | nd | nd | nd | nd | nd | nd | nd | 1.078 ± 0.02 | |
| (-)-limonene | 5989-54-8 | 1027 | 1027 | nd | nd | 12.89 ± 0.98 | 11.87 ± 15.94 | nd | nd | nd | nd | nd | |
| squalene | 111-02-4 | 2865 | 2865 | 563.30 | 510.25 | 139.59 ± 0.65 | 151.29 ± 17.3 | 311.75 ± 73.96 | 184.91 ± 27.17 | 125.47 ± 9.83 | 158.35 ± 10.83 | 75.91 ± 20.61 | |
| m-cymene | 535-77-3 | 1272 | 1270 | 10.39 | 33.08 | nd | 1.06 ± 0.30 | nd | nd | 2.44 ± 0.09 | nd | nd | |
| Terpene | limonene | 138-86-3 | 1194 | 1195 | 60.28 | 121.01 | nd | 13.01 ± 6.44 | 8.28 ± 0.50 | 4.28 ± 1.10 | 3.69 ± 1.06 | 3.41 ± 0.57 | nd |
| p-cymene | 99-87-6 | 1266 | 1268 | nd | nd | 2.74 ± 0.49 | nd | nd | nd | nd | 3.23 ± 0.91 | nd | |
| γ-terpinene | 99-85-4 | 1242 | 1242 | 1.90 | 5.59 | 1.43 ± 0.77 | 1.70 ± 0.23 | nd | nd | nd | nd | nd | |
| α-phellandrene | 99-83-2 | 1167 | 1166 | nd | nd | nd | 5.36 ± 2.48 | 2.70 ± 1.32 | 2.09 ± 0.78 | 2.05 ± 0.23 | nd | nd | |
| Δ-3-carene | 498-15-7 | 1152 | 1150 | nd | nd | nd | 9.96 ± 1.60 | nd | 3.68 ± 1.31 | nd | 4.91 ± 2.67 | 2.73 ± 0.47 | |
| Total | 635.88 | 669.93 | 156.66 | 183.89 | 322.73 | 194.97 | 131.60 | 168.77 | 78.45 | ||||
| Raw Matrix | Kombucha Fermentation Stages | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Compound | CAS | RI 1 | RI li 2 | Perception Threshold (ppm) [46] | Aroma Description [46] | Black Tea | Green Tea | D0 | D2 | D4 | D7 | D9 | D11 | D14 | |
| Carboxylic acid | A1 | 3-methylbutanoic acid | 503-74-2 | 1665 | 1664 | 50 | Cheesy, dairy, acidic, sour | nd | nd | 0.12 | 0.15 | 0.15 | 0.28 | 0.35 | 0.64 | 0.81 |
| A2 | acetic acid | 546-67-8 | 1450 | 1450 | 50.5 | Sour vinegar | nd | nd | nd | nd | nd | nd | nd | nd | 0.30 | |
| A3 | hexadecanoic acid | 57-10-3 | 2898 | 2890 | 75 | Creamy, waxy | 7.58 | 5.70 | 1.07 | 1.30 | 3.13 | 1.57 | 1.34 | 1.31 | 0.64 | |
| A4 | nonanoic acid | 112-05-0 | 2171 | 2174 | 10 | Waxy, dairy, cheesy | 1.11 | 1.67 | 0.35 | 1.44 | 1.82 | 1.37 | 1.48 | 1.39 | nd | |
| A5 | tetradecanoic acid | 544-63-8 | 2656 | 2660 | 35 | Faint, waxy, fatty | 3.84 | 3.06 | 0.51 | 0.60 | 1.86 | 0,89 | 0.63 | 0.63 | 0.25 | |
| Alcohol | B1 | geraniol | 106-24-1 | 1840 | 1836 | 3 | Floral, sweet, rosy | 14.68 | nd | 5.82 | 5.45 | 5.11 | nd | 4.63 | 4.26 | 4.03 |
| B2 | 2-phenylethanol | 60-12-8 | 1897 | 1890 | 20 | Sweet, floral, bready, honey | 0.10 | nd | 1.77 | 3.89 | 6.78 | 11.25 | 12.10 | 14.43 | 13.66 | |
| B3 | linalool | 78-70-6 | 1546 | 1545 | 10 | Citrus, floral, orange, terpeny | 22.29 | 0.54 | 7.37 | 4.58 | 3.38 | 3.84 | 4.39 | 5.31 | 4.87 | |
| B4 | 3-methylbutan-1-ol | 123-51-3 | 1216 | 1218 | 50 | Fusel, fermented, pungent | nd | nd | 0.40 | 0.54 | 0.84 | 1.33 | 1.23 | 2.31 | 2.27 | |
| Aldehyde | C1 | benzaldehyde | 100-52-7 | 1611 | 1617 | 50 | Almond, fruity, nutty | 0.21 | nd | 0.70 | nd | nd | 0.04 | 0.02 | nd | nd |
| Ester | D1 | methyl 2-hydroxybenzoate | 119-36-8 | 1764 | 1766 | 10 | Sweet, minty, root beer | 1.70 | nd | 1.72 | 1.00 | nd | nd | nd | nd | nd |
| Phenol | E1 | 4-ethyl-2-methoxyphenol | 2785-89-9 | 2016 | 2020 | 30 | Spicy, sweet vanilla, woody | nd | nd | 0.27 | 0.39 | 0.97 | 1.03 | 1.03 | 1.08 | 0.98 |
| Terpene | F1 | α-farnesene | 502-61-4 | 1687 | 1695 | 15 | Citrus, herbal, lavender, bergamot | nd | nd | nd | 10.09 | 20.78 | 12.33 | 8.36 | 10.56 | 5.06 |
| F2 | α-phellandrene | 99-83-2 | 1167 | 1166 | 20 | Citrus, herbal, terpeny, green | nd | nd | nd | 0.05 | nd | nd | 0.12 | 0.17 | nd | |
| F3 | γ-terpinene | 99-85-4 | 1242 | 1242 | 40 | Oily, woody, terpeny, tropical herbal | 0.05 | 0.14 | 0.05 | 0.32 | 0.21 | 0.11 | 0.09 | 0.08 | nd | |
| F4 | limonene | 138-86-3 | 1194 | 1195 | 30 | Citrus, orange, fresh, sweet | 2.01 | 4.03 | 0.41 | nd | 0.10 | nd | nd | nd | nd | |
| F5 | p-cymene | 99-87-6 | 1266 | 1268 | 5.5 | Woody, spicy, cumin, oregano | nd | nd | 0.43 | 1.81 | 0.56 | 0.67 | nd | nd | nd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suffys, S.; Richard, G.; Burgeon, C.; Werrie, P.-Y.; Haubruge, E.; Fauconnier, M.-L.; Goffin, D. Characterization of Aroma Active Compound Production during Kombucha Fermentation: Towards the Control of Sensory Profiles. Foods 2023, 12, 1657. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12081657
Suffys S, Richard G, Burgeon C, Werrie P-Y, Haubruge E, Fauconnier M-L, Goffin D. Characterization of Aroma Active Compound Production during Kombucha Fermentation: Towards the Control of Sensory Profiles. Foods. 2023; 12(8):1657. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12081657
Chicago/Turabian StyleSuffys, Sarah, Gaëtan Richard, Clément Burgeon, Pierre-Yves Werrie, Eric Haubruge, Marie-Laure Fauconnier, and Dorothée Goffin. 2023. "Characterization of Aroma Active Compound Production during Kombucha Fermentation: Towards the Control of Sensory Profiles" Foods 12, no. 8: 1657. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12081657