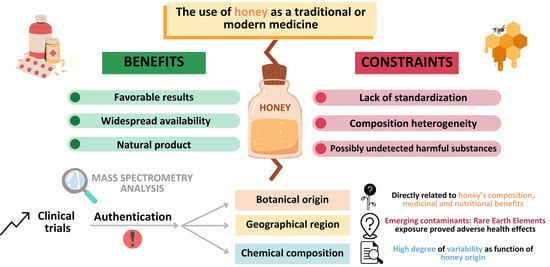
Geographical Origin Authentication—A Mandatory Step in the Efficient Involvement of Honey in Medical Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodeker, G.; Kronenberg, F.; Burford, G. Holistic, Alternative and Complementary Medicine; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Che, C.T.; George, V.; Ijinu, T.P.; Pushpangadan, P.; Andrae-Marobela, K. Traditional medicine. In Pharmacognosy; Academic Press: Cambridge, MA, USA, 2017; pp. 15–30. [Google Scholar]
- Braithwaite, M.C.; Tyagi, C.; Tomar, L.K.; Kumar, P.; Choonara, Y.E.; Pillay, V. Nutraceutical-based therapeutics and formulation strategies augmenting their efficiency to complement modern medicine: An overview. J. Funct. Foods 2014, 6, 82–99. [Google Scholar] [CrossRef]
- Van Norman, G.A. Drugs, devices, and the FDA: Part 1: An overview of approval processes for drugs. JACC Basic Transl. Sci. 2016, 1, 170–179. [Google Scholar] [CrossRef]
- Schattner, A. The spectrum of harm associated with modern medicine. J. Gen. Intern. Med. 2022, 37, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Pferschy-Wenzig, E.M.; Bauer, R. The relevance of pharmacognosy in pharmacological research on herbal medicinal products. Epilepsy Behav. 2015, 52, 344–362. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. J. Basic Med. Sci. 2013, 16, 731. [Google Scholar]
- Mijanur Rahman, M.; Gan, S.H.; Khalil, M.I. Neurological effects of honey: Current and future prospects. Evid. Based Complement. Altern. Med. 2014, 2014, 958721. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121. [Google Scholar] [CrossRef]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Afroz, R.; Tanvir, E.M.; Paul, S.; Bhoumik, N.C.; Gan, S.H.; Khalil, M.I. DNA damage inhibition properties of sundarban honey and its phenolic composition. J. Food Biochem. 2016, 40, 436–445. [Google Scholar] [CrossRef]
- Yao, L.; Datta, N.; Tomás-Barberán, F.A.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Mokaya, H.O.; Bargul, J.L.; Irungu, J.W.; Lattorff, H.M.G. Bioactive constituents, in vitro radical scavenging and antibacterial activities of selected Apis mellifera honey from Kenya. Int. J. Food Sci. Technol. 2020, 55, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Magdas, D.A.; Guyon, F.; Puscas, R.; Vigouroux, A.; Gaillard, L.; Dehelean, A.; Feher, I.; Cristea, G. Applications of emerging stable isotopes and elemental markers for geographical and varietal recognition of Romanian and French honeys. Food Chem. 2021, 334, 127599. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.; Mafra, I. A comprehensive review on the main honey authentication issues: Production and origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, N.S.; Xagoraris, M.; Revelou, P.K.; Kaparakou, E.; Kanakis, C.; Pappas, C.; Tarantilis, P. The use of SPME-GC-MS IR and Raman techniques for botanical and geographical authentication and detection of adulteration of honey. Foods 2021, 10, 1671. [Google Scholar] [CrossRef]
- Naja, G.M.; Volesky, B. Toxicity and sources of Pb, Cd, Hg, Cr, As, and radionuclides in the environment. In Handbook of Advanced Industrial and Hazardous Wastes Management; CRC Press: Boca Raton, FL, USA, 2017; pp. 855–903. [Google Scholar]
- Ungureanu, E.L.; Mocanu, A.L.; Stroe, C.A.; Duță, D.E.; Mustățea, G. Assessing Health Risks Associated with Heavy Metals in Food: A Bibliometric Analysis. Foods 2023, 12, 3974. [Google Scholar] [CrossRef] [PubMed]
- Sarsembayeva, N.B.; Abdigaliyeva, T.B.; Utepova, Z.A.; Biltebay, A.N.; Zhumagulova, S.Z. Heavy metal levels in milk and fermented milk products produced in the Almaty region, Kazakhstan. Vet. World 2020, 13, 609. [Google Scholar] [CrossRef]
- Liu, S.L.; Fan, H.R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.F.; Li, X.C. Global rare earth elements projects: New developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Yin, X.; Martineau, C.; Demers, I.; Basiliko, N.; Fenton, N.J. The potential environmental risks associated with the development of rare earth element production in Canada. Environ. Rev. 2021, 29, 354–377. [Google Scholar] [CrossRef]
- Brouziotis, A.A.; Giarra, A.; Libralato, G.; Pagano, G.; Guida, M.; Trifuoggi, M. Toxicity of rare earth elements: An overview on human health impact. Front. Environ. Sci. 2022, 10, 1617. [Google Scholar] [CrossRef]
- Wei, J.; Wang, C.; Yin, S.; Pi, X.; Jin, L.; Li, Z.; Liu, J.; Wang, L.; Yin, C.; Ren, A. Concentrations of rare earth elements in maternal serum during pregnancy and risk for fetal neural tube defects. Environ. Int. 2020, 137, 105542. [Google Scholar] [CrossRef]
- Malvandi, A.M.; Shahba, S.; Mohammadipour, A.; Rastegar-Moghaddam, S.H.; Abudayyak, M. Cell and molecular toxicity of lanthanum nanoparticles: Are there possible risks to humans? Nanotoxicology 2021, 15, 951–972. [Google Scholar] [CrossRef] [PubMed]
- Magdas, D.A.; Marincaş, O.; Cristea, G.; Feher, I.; Vedeanu, N. REEs—A possible tool for geographical origin assessment? Environ. Chem. 2019, 17, 148–157. [Google Scholar] [CrossRef]
- Sadeg, N.; Brousse, F.; Malonga, G.; Belhadj-Tahar, H. Children’s exposure to rare earth elements and cancer. Ann. Oncol. 2023, 31 (Suppl. S4), S299. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Danezis, G.P.; Halagarda, M.; Popek, S.; Georgiou, C.A. Geographical origin and botanical type honey authentication through elemental metabolomics via chemometrics. Food Chem. 2021, 338, 127936. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Casiello, G.; Kontakos, S.; Louppis, A.P.; Longobardi, F.; Kontominas, M.G. Investigating the impact of botanical origin and harvesting period on carbon stable isotope ratio values (13C/12C) and different parameter analysis of Greek unifloral honeys: A chemometric approach for correct botanical discrimination. Int. J. Food Sci. Technol. 2016, 51, 2460–2467. [Google Scholar] [CrossRef]
- Mara, A.; Deidda, S.; Caredda, M.; Ciulu, M.; Deroma, M.; Farinini, E.; Floris, I.; Langasco, I.; Leardi, R.; Pilo, M.I.; et al. Multi-Elemental Analysis as a Tool to Ascertain the Safety and the Origin of Beehive Products: Development, Validation, and Application of an ICP-MS Method on Four Unifloral Honeys Produced in Sardinia, Italy. Molecules 2022, 27, 2009. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, L.; Camin, F.; Ziller, L.; Perini, M.; Nicolini, G.; Larcher, R. Isotopic and elemental composition of selected types of Italian honey. Measurement 2017, 98, 283–289. [Google Scholar] [CrossRef]
- She, S.; Chen, L.; Song, H.; Lin, G.; Li, Y.; Zhou, J.; Liu, C. Discrimination of geographical origins of Chinese acacia honey using complex 13C/12C, oligosaccharides and polyphenols. Food Chem. 2019, 272, 580–585. [Google Scholar] [CrossRef]
- Li, C.; Kang, X.; Nie, J.; Li, A.; Farag, M.A.; Liu, C.; Rogers, K.M.; Xiao, J.; Yuan, Y. Recent advances in Chinese food authentication and origin verification using isotope ratio mass spectrometry. Food Chem. 2023, 398, 133896. [Google Scholar] [CrossRef]
- Silva, B.; Gonzaga, L.V.; Maltez, H.F.; Samochvalov, K.B.; Fett, R.; Costa, A.C.O. Elemental profiling by ICP-MS as a tool for geographical discrimination: The case of bracatinga honeydew honey. J. Food Compos. Anal. 2021, 96, 103727. [Google Scholar] [CrossRef]
- Tahboub, Y.R.; Al-Ghzawi, A.A.M.A.; Al-Zayafdneh, S.S.; AlGhotani, M.S. Levels of trace elements and rare earth elements in honey from Jordan. Environ. Sci. Pollut. Res. 2022, 29, 11469–11480. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Brizio, P.; Stella, C.; Pederiva, S.; Brusa, F.; Mogliotti, P.; Garrone, A.; Abete, M.C. Trace and rare earth elements in monofloral and multifloral honeys from Northwestern Italy; A first attempt of characterization by a multi-elemental profile. J. Trace Elem. Med. Biol. 2020, 61, 126556. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Brizio, P.; Stella, C.; Mantia, M.; Pederiva, S.; Brusa, F.; Mogliotti, P.; Garrone, A.; Abete, M.C. Trace elements and rare earth elements in honeys from the Balkans, Kazakhstan, Italy, South America, and Tanzania. Environ. Sci. Pollut. Res. 2020, 27, 12646–12657. [Google Scholar] [CrossRef] [PubMed]
- Gulino, F.; Calà, E.; Cozzani, C.; Vaccari, L.; Oddone, M.; Aceto, M. On the Traceability of Honey by Means of Lanthanide Distribution. Foods 2023, 12, 1803. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Z.; Chen, Z.; Zhang, Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 2013, 93, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Li, F.K.; Gong, A.J.; Qiu, L.N.; Zhang, W.W.; Li, J.R.; Liu, Y.; Li, J.-D.; Gao, G.; Yuan, X.T. Determination of trace rare earth elements in fruits by microwave digestion coupled with inductively coupled plasma optical emission spectrometry. Microchem. J. 2019, 147, 93–101. [Google Scholar] [CrossRef]
- Shukrimi, A.; Sulaiman, A.R.; Halim, A.Y.; Azril, A. A comparative study between honey and povidone iodine as dressing solution for Wagner type II diabetic foot ulcers. Med. J. Malays. 2008, 63, 44–46. [Google Scholar]
- Robson, V.; Yorke, J.; Sen, R.A.; Lowe, D.; Rogers, S.N. Randomised controlled feasibility trial on the use of medical grade honey following microvascular free tissue transfer to reduce the incidence of wound infection. Br. J. Oral Maxillofac. Surg. 2012, 50, 321–327. [Google Scholar] [CrossRef]
- Mphande, A.N.; Killowe, C.; Phalira, S.; Jones, H.W.; Harrison, W.J. Effects of honey and sugar dressings on wound healing. J. Wound Care 2007, 16, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Robson, V.; Dodd, S.; Thomas, S. Standardized antibacterial honey (Medihoney) with standard therapy in wound care: Randomized clinical trial. J. Adv. Nurs. 2009, 65, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.K.; Hoekstra, M.J.; Hage, J.J.; Karim, R.B. Honey-medicated dressing: Transformation of an ancient remedy into modern therapy. Ann. Plast. Surg. 2003, 50, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, M. Early tangential excision and skin grafting of moderate burns is superior to honey dressing: A prospective randomised trial. Burns 1999, 25, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.A.; Halim, A.S.; Singh, K.K.; Dorai, A.A.; Haneef, M.N. Antibacterial properties of tualang honey and its effect in burn wound management: A comparative study. BMC Complement. Altern. Med. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.; Müller, M.C.; Binnekade, J.M.; van den Akker, J.P.; de Borgie, C.A.; Schultz, M.J.; Zaat, S.A. Medical-grade honey does not reduce skin colonization at central venous catheter-insertion sites of critically ill patients: A randomized controlled trial. Crit. Care 2012, 16, R214. [Google Scholar] [CrossRef] [PubMed]
- Misirlioglu, A.; Eroglu, S.; Karacaoglan, N.; Akan, M.; Akoz, T.; Yildirim, S. Use of honey as an adjunct in the healing of split-thickness skin graft donor site. Dermatol. Surg. 2003, 29, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, M. Honey dressing versus boiled potato peel in the treatment of burns: A prospective randomized study. Burns 1996, 22, 491–493. [Google Scholar] [CrossRef]
- Okeniyi, J.A.; Olubanjo, O.O.; Ogunlesi, T.A.; Oyelami, O.A. Comparison of healing of incised abscess wounds with honey and EUSOL dressing. J. Altern. Complement. Med. 2005, 11, 511–513. [Google Scholar] [CrossRef]
- Malik, K.I.; Malik, M.A.; Aslam, A. Honey compared with silver sulphadiazine in the treatment of superficial partial-thickness burns. Int. Wound J. 2010, 7, 413–417. [Google Scholar] [CrossRef]
- Lund-Nielsen, B.; Adamsen, L.; Gottrup, F.; Rorth, M.; Tolver, A.; Kolmos, H.J. Qualitative bacteriology in malignant wounds—A prospective, randomized, clinical study to compare the effect of honey and silver dressings. Ostomy Wound Manag. 2011, 57, 28–36. [Google Scholar]
- Gethin, G.; Cowman, S. Bacteriological changes in sloughy venous leg ulcers treated with manuka honey or hydrogel: An RCT. J. Wound Care 2008, 17, 241–247. [Google Scholar] [CrossRef]
- Alasqah, M.; Alrashidi, A.; Alshammari, N.; Alshehri, A.; Gufran, K. Effect of honey dressing material on palatal wound healing after harvesting a free gingival graft: A prospective randomized case control study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2662–2668. [Google Scholar] [CrossRef]
- Kamaratos, A.V.; Tzirogiannis, K.N.; Iraklianou, S.A.; Panoutsopoulos, G.I.; Kanellos, I.E.; Melidonis, A.I. Manuka honey-impregnated dressings in the treatment of neuropathic diabetic foot ulcers. Int. Wound J. 2014, 11, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Yapucu Güneş, U.; Eşer, I. Effectiveness of a honey dressing for healing pressure ulcers. J. Wound Ostomy Cont. Nurs. 2007, 34, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Biglari, B.; vd Linden, P.H.; Simon, A.; Aytac, S.; Gerner, H.J.; Moghaddam, A. Use of Medihoney as a non-surgical therapy for chronic pressure ulcers in patients with spinal cord injury. Spinal Cord 2012, 50, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Ziahosseini, K.; Poitelea, C.; Litwin, A.; Sagili, S. Effect of Manuka Honey on Eyelid Wound Healing: A Randomized Controlled Trial. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Alvarez, A.; Massey, C.; Miller, L.; Rannou, R.; Croce, M.; Alleman, T. Novel bioengineered collagen with Manuka honey and hydroxyapatite sheet for the treatment of lower extremity chronic wounds in an urban hospital wound care setting. Wounds 2023, 35, E35–E38. [Google Scholar] [CrossRef] [PubMed]
- Henatsch, D.; Wesseling, F.; Briedé, J.J.; Stokroos, R.J. Treatment of chronically infected open mastoid cavities with medical honey: A randomized controlled trial. Otol. Neurotol. 2015, 36, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Dunford, C.E.; Hanano, R. Acceptability to patients of a honey dressing for non-healing venous leg ulcers. J. Wound Care 2004, 13, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Moghazy, A.M.; Shams, M.E.; Adly, O.A.; Abbas, A.H.; El-Badawy, M.A.; Elsakka, D.M.; Hassan, S.A.; Abdelmohsen, W.S.; Ali, O.S.; Mohamed, B.A. The clinical and cost effectiveness of bee honey dressing in the treatment of diabetic foot ulcers. Diabetes Res. Clin. Pract. 2010, 89, 276–281. [Google Scholar] [CrossRef]
- Schumacher, H.H. Use of medical honey in patients with chronic venous leg ulcers after split-skin grafting. J. Wound Care 2004, 13, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; van Eps, C.; Mudge, D.W.; Wiggins, K.J.; Armstrong, K.; Hawley, C.M.; Campbell, S.B.; Isbel, N.M.; Nimmo, G.R.; Gibbs, H. Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, M. Honey-impregnated gauze versus amniotic membrane in the treatment of burns. Burns 1994, 20, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, M. Honey impregnated gauze versus polyurethane film (OpSite) in the treatment of burns—A prospective randomised study. Br. J. Plast. Surg. 1993, 46, 322–323. [Google Scholar] [CrossRef]
- Yousaf, I.; Ishaq, I.; Hussain, M.B.; Inaam, S.; Saleem, S.; Qamar, M.U. Antibacterial activity of Pakistani Beri honey compared with silver sulfadiazine on infected wounds: A clinical trial. J. Wound Care 2019, 28, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Chotchoungchatchai, S.; Krairit, O.; Tragulpiankit, P.; Prathanturarug, S. The efficacy of honey and a Thai Herbal Oil preparation in the treatment of pressure ulcers based on Thai traditional medicine wound diagnosis versus standard practice: An open-label randomized controlled trial. Contemp. Clin. Trials Commun. 2020, 17, 100538. [Google Scholar] [CrossRef] [PubMed]
- Jull, A.; Walker, N.; Parag, V.; Molan, P.; Rodgers, A. Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br. J. Surg. 2008, 95, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Badve, S.V.; Pascoe, E.M.; Beller, E.; Cass, A.; Clark, C.; de Zoysa, J.; Isbel, N.M.; McTaggart, S.; Morrish, A.T.; et al. Antibacterial honey for the prevention of peritoneal-dialysis-related infections (HONEYPOT): A randomised trial. Lancet Infect. Dis. 2014, 14, 23–30. [Google Scholar] [CrossRef]
- Zhang, L.; Badve, S.V.; Pascoe, E.M.; Beller, E.; Cass, A.; Clark, C.; de Zoysa, J.; Isbel, N.M.; McTaggart, S.; Morrish, A.T.; et al. The Effect of Exit-Site Antibacterial Honey Versus Nasal Mupirocin Prophylaxis on the Microbiology and Outcomes of Peritoneal Dialysis-Associated Peritonitis and Exit-Site Infections: A Sub-Study of the Honeypot Trial. Perit. Dial. Int. 2015, 35, 712–721. [Google Scholar] [CrossRef]
- Lund-Nielsen, B.; Adamsen, L.; Kolmos, H.J.; Rørth, M.; Tolver, A.; Gottrup, F. The effect of honey-coated bandages compared with silver-coated bandages on treatment of malignant wounds-a randomized study. Wound Repair Regen. 2011, 19, 664–670. [Google Scholar] [CrossRef]
- Gourdomichali, T.; Papakonstantinou, E. Short-term effects of six Greek honey varieties on glycemic response: A randomized clinical trial in healthy subjects. Eur. J. Clin. Nutr. 2018, 72, 1709–1716. [Google Scholar] [CrossRef]
- Münstedt, K.; Sheybani, B.; Hauenschild, A.; Brüggmann, D.; Bretzel, R.G.; Winter, D. Effects of basswood honey, honey-comparable glucose-fructose solution, and oral glucose tolerance test solution on serum insulin, glucose, and C-peptide concentrations in healthy subjects. J. Med. Food 2008, 11, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Mazruei Arani, N.; Emam-Djomeh, Z.; Tavakolipour, H.; Sharafati-Chaleshtori, R.; Soleimani, A.; Asemi, Z. The Effects of Probiotic Honey Consumption on Metabolic Status in Patients with Diabetic Nephropathy: A Randomized, Double-Blind, Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Deibert, P.; König, D.; Kloock, B.; Groenefeld, M.; Berg, A. Glycaemic and insulinaemic properties of some German honey varieties. Eur. J. Clin. Nutr. 2010, 64, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, P.; Parry-Strong, A.; Walsh, E.; Weatherall, M.; Krebs, J.D. The effect of a cinnamon-, chromium- and magnesium-formulated honey on glycaemic control, weight loss and lipid parameters in type 2 diabetes: An open-label cross-over randomised controlled trial. Eur. J. Nutr. 2016, 55, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Chlup, R.; Peterson, K.; Zapletalová, J.; Kudlová, P.; Seckar, P. Extended prandial glycemic profiles of foods as assessed using continuous glucose monitoring enhance the power of the 120-min glycemic index. J. Diabetes Sci. Technol. 2010, 4, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, W.S.; Romli, A.C.; Mohamed, M. Effects of honey supplementation on inflammatory markers among chronic smokers: A randomized controlled trial. BMC Complement. Altern. Med. 2017, 17, 175. [Google Scholar] [CrossRef]
- Abbey, E.L.; Rankin, J.W. Effect of ingesting a honey-sweetened beverage on soccer performance and exercise-induced cytokine response. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 659–672. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Abdul Aziz, A.; Kong, K.W.; Hamid, M.S.A.; Cheong, J.P.G.; Hamzah, S.H. Dose-Response Effect of Tualang Honey on Postprandial Antioxidant Activity and Oxidative Stress in Female Athletes: A Pilot Study. J. Altern. Complement. Med. 2017, 23, 989–995. [Google Scholar] [CrossRef]
- Abdulrhman, M.; El-Hefnawy, M.; Hussein, R.; El-Goud, A.A. The glycemic and peak incremental indices of honey, sucrose and glucose in patients with type 1 diabetes mellitus: Effects on C-peptide level-a pilot study. Acta Diabetol. 2011, 48, 89–94. [Google Scholar] [CrossRef]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Willis, K.S.; Willis, L.M.; Austin, K.J.; Hart, A.M.; Breton, A.B.; Alexander, B.M. Effect of honey versus sucrose on appetite, appetite-regulating hormones, and postmeal thermogenesis. J. Am. Coll. Nutr. 2010, 29, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Fetzner, L.; Burhenne, J.; Weiss, J.; Völker, M.; Unger, M.; Mikus, G.; Haefeli, W.E. Daily honey consumption does not change CYP3A activity in humans. J. Clin. Pharmacol. 2011, 51, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Ab Wahab, S.Z.; Hussain, N.H.N.; Zakaria, R.; Kadir, A.A.; Mohamed, N.; Tohit, N.M.; Norhayati, M.N.; Hassan, I.I. Long-term effects of honey on cardiovascular parameters and anthropometric measurements of postmenopausal women. Complement. Ther. Med. 2018, 41, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.S. Natural honey lowers plasma glucose, C-reactive protein, homocysteine, and blood lipids in healthy, diabetic, and hyperlipidemic subjects: Comparison with dextrose and sucrose. J. Med. Food 2004, 7, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.Y.; Nassar, M.F.; Ezz El-Arab, S.; Henein, H.H. Effect of honey supplementation on the phagocytic function during nutritional rehabilitation of protein energy malnutrition patients. J. Trop. Pediatr. 2012, 58, 159–160. [Google Scholar] [CrossRef]
- Yaghoobi, N.; Al-Waili, N.; Ghayour-Mobarhan, M.; Parizadeh, S.M.; Abasalti, Z.; Yaghoobi, Z.; Yaghoobi, F.; Esmaeili, H.; Kazemi-Bajestani, S.M.; Aghasizadeh, R.; et al. Natural honey and cardiovascular risk factors; effects on blood glucose, cholesterol, triacylglycerole, CRP, and body weight compared with sucrose. Sci. World J. 2008, 8, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Buckwheat honey increases serum antioxidant capacity in humans. J. Agric. Food Chem. 2003, 51, 1500–1505. [Google Scholar] [CrossRef]
- Farakla, I.; Koui, E.; Arditi, J.; Papageorgiou, I.; Bartzeliotou, A.; Papadopoulos, G.E.; Mantzou, A.; Papathanasiou, C.; Dracopoulou, M.; Papastamataki, M.; et al. Effect of honey on glucose and insulin concentrations in obese girls. Eur. J. Clin. Investig. 2019, 49, e13042. [Google Scholar] [CrossRef]
- Rasad, H.; Entezari, M.H.; Ghadiri, E.; Mahaki, B.; Pahlavani, N. The effect of honey consumption compared with sucrose on lipid profile in young healthy subjects (randomized clinical trial). Clin. Nutr. ESPEN 2018, 26, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Zuhri, M.I.M.; Mohamed, M.M.D.; Kiew, O.F.; Ahmad, N.S. Combination of Honey Supplementation and Countermovement Jumping Exercise Influence Bone Turnover Markers in Young Males. Sains Malays. 2020, 49, 1639–1649. [Google Scholar] [CrossRef]
- Al-Tamimi, A.M.; Petrisko, M.; Hong, M.Y.; Rezende, L.; Clayton, Z.S.; Kern, M. Honey does not adversely impact blood lipids of adult men and women: A randomized cross-over trial. Nutr. Res. 2020, 74, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Abdulrhman, M.A.; Nassar, M.F.; Mostafa, H.W.; El-Khayat, Z.A.; Abu El Naga, M.W. Effect of honey on 50% complement hemolytic activity in infants with protein energy malnutrition: A randomized controlled pilot study. J. Med. Food 2011, 14, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Abdulrhman, M.; El Hefnawy, M.; Ali, R.; Abdel Hamid, I.; Abou El-Goud, A.; Refai, D. Effects of honey, sucrose and glucose on blood glucose and C-peptide in patients with type 1 diabetes mellitus. Complement Ther. Clin. Pract. 2013, 19, 15–19. [Google Scholar] [CrossRef]
- Abdulrhman, M.M.; El-Hefnawy, M.H.; Aly, R.H.; Shatla, R.H.; Mamdouh, R.M.; Mahmoud, D.M.; Mohamed, W.S. Metabolic effects of honey in type 1 diabetes mellitus: A randomized crossover pilot study. J. Med. Food 2013, 16, 66–72. [Google Scholar] [CrossRef]
- Münstedt, K.; Hoffmann, S.; Hauenschild, A.; Bülte, M.; von Georgi, R.; Hackethal, A. Effect of honey on serum cholesterol and lipid values. J. Med. Food 2009, 12, 624–628. [Google Scholar] [CrossRef]
- Bhatti, I.; Inayat, S.; Uzair, B.; Menaa, F.; Bakhsh, S.; Khan, H.; Naz, F.; Khan, B.A. Effects of Nigella sativa (Kalonji) and honey on lipid profile of hyper lipidemic smokers. Ind. J. Pharmaceut. Educ. Res. 2016, 50, 376–384. [Google Scholar] [CrossRef]
- Saarinen, K.; Jantunen, J.; Haahtela, T. Birch pollen honey for birch pollen allergy—A randomized controlled pilot study. Int. Arch. Allergy Immunol. 2011, 155, 160–166. [Google Scholar] [CrossRef]
- Wallace, A.; Eady, S.; Miles, M.; Martin, H.; McLachlan, A.; Rodier, M.; Willis, J.; Scott, R.; Sutherland, J. Demonstrating the safety of manuka honey UMF 20+in a human clinical trial with healthy individuals. Br. J. Nutr. 2010, 103, 1023–1028. [Google Scholar] [CrossRef]
- Onyesom, I. Honey-induced stimulation of blood ethanol elimination and its influence on serum triacylglycerol and blood pressure in man. Ann. Nutr. Metab. 2005, 49, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Tushar, T.; Vinod, T.; Rajan, S.; Shashindran, C.; Adithan, C. Effect of honey on CYP3A4, CYP2D6 and CYP2C19 enzyme activity in healthy human volunteers. Basic Clin. Pharmacol. Toxicol. 2007, 100, 269–272. [Google Scholar] [CrossRef]
- Desfita, S.; Sari, W.; Yusmarini, Y.; Pato, U.; Zakłos-Szyda, M.; Budryn, G. Effect of Fermented Soymilk-Honey from Different Probiotics on Osteocalcin Level in Menopausal Women. Nutrients 2021, 13, 3581. [Google Scholar] [CrossRef]
- Bahrami, M.; Ataie-Jafari, A.; Hosseini, S.; Foruzanfar, M.H.; Rahmani, M.; Pajouhi, M. Effects of natural honey consumption in diabetic patients: An 8-week randomized clinical trial. Int. J. Food Sci. Nutr. 2009, 60, 618–626. [Google Scholar] [CrossRef]
- Bardy, J.; Molassiotis, A.; Ryder, W.D.; Mais, K.; Sykes, A.; Yap, B.; Lee, L.; Kaczmarski, E.; Slevin, N. A double-blind, placebo-controlled, randomised trial of active manuka honey and standard oral care for radiation-induced oral mucositis. Br. J. Oral Maxillofac Surg. 2012, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Shoma, A.; Eldars, W.; Noman, N.; Saad, M.; Elzahaf, E.; Abdalla, M.; Eldin, D.S.; Zayed, D.; Shalaby, A.; Malek, H.A. Pentoxifylline and local honey for radiation-induced burn following breast conservative surgery. Curr. Clin. Pharmacol. 2010, 5, 251–256. [Google Scholar] [CrossRef]
- Rashad, U.M.; Al-Gezawy, S.M.; El-Gezawy, E.; Azzaz, A.N. Honey as topical prophylaxis against radiochemotherapy-induced mucositis in head and neck cancer. J. Laryngol. Otol. 2009, 123, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar, M.; Poorter, R.L.; van der Toorn, P.P.; Lenderink, A.W.; Poortmans, P.; Egberts, A.C. The effect of honey compared to conventional treatment on healing of radiotherapy-induced skin toxicity in breast cancer patients. Acta Oncol. 2006, 45, 623–624. [Google Scholar] [CrossRef]
- Charalambous, A.; Lambrinou, E.; Katodritis, N.; Vomvas, D.; Raftopoulos, V.; Georgiou, M.; Paikousis, L.; Charalambous, M. The effectiveness of thyme honey for the management of treatment-induced xerostomia in head and neck cancer patients: A feasibility randomized control trial. Eur. J. Oncol. Nurs. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Khanjani Pour-Fard-Pachekenari, A.; Rahmani, A.; Ghahramanian, A.; Asghari Jafarabadi, M.; Onyeka, T.C.; Davoodi, A. The effect of an oral care protocol and honey mouthwash on mucositis in acute myeloid leukemia patients undergoing chemotherapy: A single-blind clinical trial. Clin. Oral Investig. 2019, 23, 1811–1821. [Google Scholar] [CrossRef]
- Biswal, B.M.; Zakaria, A.; Ahmad, N.M. Topical application of honey in the management of radiation mucositis: A preliminary study. Support Care Cancer 2003, 11, 242–248. [Google Scholar] [CrossRef]
- Hawley, P.; Hovan, A.; McGahan, C.E.; Saunders, D. A randomized placebo-controlled trial of manuka honey for radiation-induced oral mucositis. Support Care Cancer 2014, 22, 751–761. [Google Scholar] [CrossRef]
- Abdulrhman, M.; Elbarbary, N.S.; Ahmed Amin, D.; Saeid Ebrahim, R. Honey and a mixture of honey, beeswax, and olive oil-propolis extract in treatment of chemotherapy-induced oral mucositis: A randomized controlled pilot study. Pediatr. Hematol. Oncol. 2012, 29, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Khanal, B.; Baliga, M.; Uppal, N. Effect of topical honey on limitation of radiation-induced oral mucositis: An intervention study. Int. J. Oral Maxillofac. Surg. 2010, 39, 1181–1185. [Google Scholar] [CrossRef]
- Sahebnasagh, M.; Aksi, V.; Eslami, F.; Lashkardoost, H.; Kasaian, J.; Golmohammadzadeh, S.; Parkam, B.; Negarandeh, R.; Saghafi, F.; Sahebnasagh, A. Prevention of radiotherapy-related oral mucositis with zinc and polyherbal mouthwash: A double-blind, randomized clinical trial. Eur. J. Med. Res. 2023, 28, 109. [Google Scholar] [CrossRef]
- Badr, L.K.; El Asmar, R.; Hakim, S.; Saad, R.; Merhi, R.; Zahreddine, A.; Muwakkit, S. The efficacy of honey or olive oil on the severity of oral mucositis and pain compared to placebo (standard care) in children with leukemia receiving intensive chemotherapy: A randomized controlled trial (RCT). J. Pediatr. Nurs. 2023, 70, e48–e53. [Google Scholar] [CrossRef] [PubMed]
- Fogh, S.E.; Deshmukh, S.; Berk, L.B.; Dueck, A.C.; Roof, K.; Yacoub, S.; Gergel, T.; Stephans, K.; Rimner, A.; DeNittis, A.; et al. A Randomized Phase 2 Trial of Prophylactic Manuka Honey for the Reduction of Chemoradiation Therapy-Induced Esophagitis During the Treatment of Lung Cancer: Results of NRG Oncology RTOG 1012. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Zidan, J.; Shetver, L.; Gershuny, A.; Abzah, A.; Tamam, S.; Stein, M.; Friedman, E. Prevention of chemotherapy-induced neutropenia by special honey intake. Med. Oncol. 2006, 23, 549–552. [Google Scholar] [CrossRef]
- Charalambous, M.; Raftopoulos, V.; Paikousis, L.; Katodritis, N.; Lambrinou, E.; Vomvas, D.; Georgiou, M.; Charalambous, A. The effect of the use of thyme honey in minimizing radiation-induced oral mucositis in head and neck cancer patients: A randomized controlled trial. Eur. J. Oncol. Nurs. 2018, 34, 89–97. [Google Scholar] [CrossRef]
- Abdulrhman, M.A.; Hamed, A.A.; Mohamed, S.A.; Hassanen, N.A. Effect of honey on febrile neutropenia in children with acute lymphoblastic leukemia: A randomized crossover open-labeled study. Complement. Ther. Med. 2016, 25, 98–103. [Google Scholar] [CrossRef]
- Raeessi, M.A.; Raeessi, N.; Panahi, Y.; Gharaie, H.; Davoudi, S.M.; Saadat, A.; Karimi Zarchi, A.A.; Raeessi, F.; Ahmadi, S.M.; Jalalian, H. “Coffee plus honey” versus “topical steroid” in the treatment of chemotherapy-induced oral mucositis: A randomised controlled trial. BMC Complement. Altern. Med. 2014, 14, 293. [Google Scholar] [CrossRef]
- Lee, V.S.; Humphreys, I.M.; Purcell, P.L.; Davis, G.E. Manuka honey versus saline sinus irrigation in the treatment of cystic fibrosis-associated chronic rhinosinusitis: A randomised pilot trial. Clin. Otolaryngol. 2021, 46, 168–174. [Google Scholar] [CrossRef]
- Poovelikunnel, T.T.; Gethin, G.; Solanki, D.; McFadden, E.; Codd, M.; Humphreys, H. Randomized controlled trial of honey versus mupirocin to decolonize patients with nasal colonization of meticillin-resistant Staphylococcus aureus. J. Hosp. Inf. 2018, 98, 141–148. [Google Scholar] [CrossRef]
- Ooi, M.L.; Jothin, A.; Bennett, C.; Ooi, E.H.; Vreugde, S.; Psaltis, A.J.; Wormald, P.J. Manuka honey sinus irrigations in recalcitrant chronic rhinosinusitis: Phase 1 randomized, single-blinded, placebo-controlled trial. Int. Forum Allergy. Rhinol. 2019, 9, 1470–1477. [Google Scholar] [CrossRef]
- Lee, V.S.; Humphreys, I.M.; Purcell, P.L.; Davis, G.E. Manuka honey sinus irrigation for the treatment of chronic rhinosinusitis: A randomized controlled trial. Int. Forum Allergy Rhinol. 2017, 7, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, F.; Baghbanian, N.; Majd, Z.; Rouini, M.R.; Jahanshahi, J.; Hashemian, F. The effect of thyme honey nasal spray on chronic rhinosinusitis: A double-blind randomized controlled clinical trial. Eur. Arch. Otorhinolaryngol. 2015, 272, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, D.M.; Rizzo, J.A.; Schor, D.; Silva, A.R.; Oliveira, D.C.; Solé, D.; Sarinho, E. Use of honey associated with Ananas comosus (Bromelin) in the treatment of acute irritative cough. Uso do mel de abelha associado ao Ananas comosus (Bromelin) no tratamento da tosse irritativa aguda. Rev. Paul. Pediatr. 2016, 34, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A.; Gensler, G.; Robbins, J.; Lindblad, A.S.; Brandt, D.; Hind, J.A.; Kosek, S.; Dikeman, K.; Kazandjian, M.; Gramigna, G.D.; et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. J. Speech Lang. Hear. Res. 2008, 51, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Moragas, A.; Ouchi, D.; Monfà, R.; Garcia-Sangenís, A.; Gómez-Lumbreras, A.; Pera, H.; Pujol, J.; Morros, R. Effectiveness of antitussives, anticholinergics, and honey versus usual care in adults with uncomplicated acute bronchitis: A multiarm randomized clinical trial. Fam. Pract. 2023, 40, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.V.; Tennen, H.; Lindquist, R.L.; Cohen, L.; Clive, J. Effect of ingestion of honey on symptoms of rhinoconjunctivitis. Ann. Allergy Asthma Immunol. 2002, 88, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Miceli Sopo, S.; Greco, M.; Monaco, S.; Varrasi, G.; Di Lorenzo, G.; Simeone, G.; Milk Honey Study (MHS) Group. Effect of multiple honey doses on non-specific acute cough in children. An open randomised study and literature review. Allergol. Immunopathol 2015, 43, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Asha’ari, Z.A.; Ahmad, M.Z.; Jihan, W.S.; Che, C.M.; Leman, I. Ingestion of honey improves the symptoms of allergic rhinitis: Evidence from a randomized placebo-controlled trial in the East coast of Peninsular Malaysia. Ann. Saudi Med. 2013, 33, 469–475. [Google Scholar] [CrossRef]
- Robbins, J.; Gensler, G.; Hind, J.; Logemann, J.A.; Lindblad, A.S.; Brandt, D.; Baum, H.; Lilienfeld, D.; Kosek, S.; Lundy, D.; et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: A randomized trial. Ann. Intern. Med. 2008, 148, 509–518. [Google Scholar] [CrossRef]
- Carnevali, I.; La Paglia, R.; Pauletto, L.; Raso, F.; Testa, M.; Mannucci, C.; Sorbara, E.E.; Calapai, G. Efficacy and safety of the syrup “KalobaTUSS®” as a treatment for cough in children: A randomized, double blind, placebo-controlled clinical trial. BMC Pediatr. 2021, 21, 29. [Google Scholar] [CrossRef]
- Semprini, A.; Singer, J.; Braithwaite, I.; Shortt, N.; Thayabaran, D.; McConnell, M.; Weatherall, M.; Beasley, R. Kanuka honey versus aciclovir for the topical treatment of herpes simplex labialis: A randomised controlled trial. BMJ Open 2019, 9, e026201. [Google Scholar] [CrossRef] [PubMed]
- Halimeh, A.; Farhad, R.B.; Naseh, S.; Karim, N. Comparative efficacy of honey 12.5% and chlorhexidine 0.2% mouthwashes on the oropharyngeal bacterial colonization in mechanically-ventilated patients: A randomized controlled trial. J. Tradit. Chin. Med. 2020, 40, 440–446. [Google Scholar] [CrossRef] [PubMed]
- El-Haddad, S.A.; Asiri, F.Y.; Al-Qahtani, H.H.; Al-Ghmlas, A.S. Efficacy of honey in comparison to topical corticosteroid for treatment of recurrent minor aphthous ulceration: A randomized, blind, controlled, parallel, double-center clinical trial. Quintessence Int. 2014, 45, 691–701. [Google Scholar] [CrossRef]
- Konuk Sener, D.; Aydin, M.; Cangur, S.; Guven, E. The Effect of Oral Care with Chlorhexidine, Vitamin E and Honey on Mucositis in Pediatric Intensive Care Patients: A Randomized Controlled Trial. J. Pediatr. Nurs. 2019, 45, e95–e101. [Google Scholar] [CrossRef]
- Sreenivasan, P.K.; Kakarla, V.V.P.; Sharda, S.; Setty, Y. The effects of a novel herbal toothpaste on salivary lactate dehydrogenase as a measure of cellular integrity. Clin. Oral. Investig. 2021, 25, 3021–3030. [Google Scholar] [CrossRef]
- Nandlal, B.; Sreenivasan, P.K.; Shashikumar, P.; Devishree, G.; Bettahalli Shivamallu, A. A randomized clinical study to examine the oral hygiene efficacy of a novel herbal toothpaste with zinc over a 6-month period. Int. J. Dent. Hyg. 2021, 19, 440–449. [Google Scholar] [CrossRef]
- Altaweel, A.A.; El-Hamid Gaber, A.; Alnaffar, M.Z.; Almowallad, A.S.; Almech, M.H.; Almuwallad, A.S.; Alharbi, R.K.; Arab, W.A. A novel therapeutic approach for reducing postoperative inflammatory complications after impacted mandibular third molar removal. Medicine 2022, 101, e30436. [Google Scholar] [CrossRef]
- Mhapuskar, A.A.; Thakare, S.; Kale, I.P.; Karmarkar, P.; Hiremutt, D.R.P.; Jadhav, A. Comparison of effects of honey and 0.1% triamcinolone acetonide in the management of recurrent aphthous stomatitis—A randomised, single-blind study. J. Ind. Acad. Oral Med. Radiol. 2022, 34, 276–280. [Google Scholar]
- Anggraeni, D.T.; Hayati, A.T.; Nur’aeni, A. The effect of oral care using honey as an additional topical agent on oral health status of intubated patients in the intensive care unit. Enfermería Intensiv. Eng. Ed. 2022, 33, 225–232. [Google Scholar] [CrossRef]
- Onuoha, E.O.; Adekunle, A.A.; Ajike, S.O.; Gbotolorun, O.M.; Adeyemo, W.L. Effect of manuka honey socket dressing on postoperative sequelae and complications following third molar extraction: A randomized controlled study. J. Craniomaxillofac. Surg. 2023, 51, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Singh, V.; Lukram, A.; Chatterjee, S.; Khan, A.M.; Dawar, G. Antibacterial efficacy of manuka honey, ocimum sanctum, curcuma longa and 0.2% chlorhexidine mouthwash on the level of streptococcus mutans and lactobacillus acidophilus—A randomized controlled trial. Indian J. Dent. Res. 2022, 33, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Janiani, P.; Gurunathan, D. Effectiveness of pre-administered natural sweet-tasting solution for decreasing pain associated with dental injections in children: A split-mouth randomized controlled trial. J. Contemp. Dent. Pract. 2021, 22, 1434–1437. [Google Scholar] [PubMed]
- Abdel-Naby Awad, O.G.; Hamad, A.H. Honey can help in herpes simplex gingivostomatitis in children: Prospective randomized double blind placebo controlled clinical trial. Am. J. Otolaryngol. 2018, 39, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.; Abou-Bakr, A.; Ghalwash, D.M.; Hussein, R.R. Effectiveness of thyme honey in the management of xerostomia in geriatric patients with end-stage renal disease: A randomized controlled clinical trial with a biochemical assessment. Eur. J. Med. Res. 2023, 28, 406. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, G.; Ramli, R.R.; Singh, H.; Abdullah, B. Tualang honey versus steroid impregnated nasal dressing following endoscopic sinus surgery: A randomized controlled trial. J. Complement. Integr. Med. 2020, 18, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Geißler, K.; Schulze, M.; Inhestern, J.; Meißner, W.; Guntinas-Lichius, O. The effect of adjuvant oral application of honey in the management of postoperative pain after tonsillectomy in adults: A pilot study. PLoS ONE 2020, 15, e0228481. [Google Scholar] [CrossRef]
- Lubis, A.S.; Herwanto, H.R.Y.; Rambe, A.Y.M.; Munir, D.; Asroel, H.A.; Ashar, T.; Lelo, A. The effect of honey on post-tonsillectomy pain relief: A randomized clinical trial. Braz. J. Otorhinolaryngol. 2023, 89, 60–65. [Google Scholar] [CrossRef]
- McIntosh, C.D.; Thomson, C.E. Honey dressing versus paraffin tulle gras following toenail surgery. J. Wound Care 2016, 15, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Movahed, R.; Rajabi, O.; Azizi, H.; Jafari, S.; Yousefi, R.; Bakhshaee, M. The effect of standardized honey on mucosal healing of the nose and paranasal sinuses after polypectomy: A randomized controlled, double blind pilot study. Acta Biomed. 2022, 92, e2021293. [Google Scholar] [CrossRef]
- Goharshenasan, P.; Amini, S.; Atria, A.; Abtahi, H.; Khorasani, G. Topical Application of Honey on Surgical Wounds: A Randomized Clinical Trial. Forsch Komplementmed. 2016, 23, 12–15. [Google Scholar] [CrossRef]
- Mat Lazim, N.; Abdullah, B.; Salim, R. The effect of Tualang honey in enhancing post tonsillectomy healing process. An open labelled prospective clinical trial. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, B.; Lazim, N.M.; Salim, R. The effectiveness of Tualang honey in reducing post-tonsillectomy pain. Kulak Burun Bogaz Ihtis Derg. 2015, 25, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Mirjalili, M.; Ayatollahi, V.; Vaziribozorg, S.; Zand, V. Comparing the Efficacy of Peritonsillar Injection of Tramadol with Honey in Controlling Post-Tonsillectomy Pain in Adults. J. Craniofac. Surg. 2018, 29, e384–e387. [Google Scholar] [CrossRef] [PubMed]
- Ozlugedik, S.; Genc, S.; Unal, A.; Elhan, A.H.; Tezer, M.; Titiz, A. Can postoperative pains following tonsillectomy be relieved by honey? A prospective, randomized, placebo controlled preliminary study. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, S.; Nia, F.H.; Kelantari, F.; Nejad, S.E.; Hamedi, Y.; Abd, R. Efficacy of honey in reduction of post tonsillectomy pain, randomized clinical trial. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1886–1889. [Google Scholar] [CrossRef]
- Razdar, S.; Panahi, Y.; Mohammadi, R.; Khedmat, L.; Khedmat, H. Evaluation of the efficacy and safety of an innovative flavonoid lotion in patients with haemorrhoid: A randomised clinical trial. BMJ Open Gastroenterol. 2023, 10, e001158. [Google Scholar] [CrossRef]
- Ladas, S.D.; Haritos, D.N.; Raptis, S.A. Honey may have a laxative effect on normal subjects because of incomplete fructose absorption. Am. J. Clin. Nutr. 1995, 62, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Hefner, J.N.; Howard, R.S.; Massey, R.; Valencia, M.; Stocker, D.J.; Philla, K.Q.; Goldman, M.D.; Nylund, C.M.; Min, S.B. A Randomized Controlled Comparison of Esophageal Clearance Times of Oral Budesonide Preparations. Dig. Dis. Sci. 2016, 61, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Andayani, R.P.; Nurhaeni, N.; Agustini, N. The Effect of Honey with ORS and a Honey Solution in ORS on Reducing the Frequency of Diarrhea and Length of Stay for Toddlers. Compr. Child Adolesc. Nurs. 2019, 42 (Suppl. S1), 21–28. [Google Scholar] [CrossRef]
- Mohtashami, R.; Huseini, H.F.; Heydari, M.; Amini, M.; Sadeqhi, Z.; Ghaznavi, H.; Mehrzadi, S. Efficacy and safety of honey based formulation of Nigella sativa seed oil in functional dyspepsia: A double blind randomized controlled clinical trial. J. Ethnopharmacol. 2015, 175, 147–152. [Google Scholar] [CrossRef]
- Shaaban, S.Y.; Abdulrhman, M.A.; Nassar, M.F.; Fathy, R.A. Effect of honey on gastric emptying of infants with protein energy malnutrition. Eur. J. Clin. Investig. 2010, 40, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Abdulrhman, M.A.; Mekawy, M.A.; Awadalla, M.M.; Mohamed, A.H. Bee honey added to the oral rehydration solution in treatment of gastroenteritis in infants and children. J. Med. Food 2010, 13, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Hashem-Dabaghian, F.; Agah, S.; Taghavi-Shirazi, M.; Ghobadi, A. Combination of Nigella sativa and Honey in Eradication of Gastric Helicobacter pylori Infection. Iran. Red Crescent Med. J. 2016, 18, e23771. [Google Scholar] [CrossRef]
- Darvishi, M.; Jahdi, F.; Hamzegardeshi, Z.; Goodarzi, S.; Vahedi, M. The Comparison of vaginal cream of mixing yogurt, honey and clotrimazole on symptoms of vaginal candidiasis. Glob. J. Health Sci. 2015, 7, 108–116. [Google Scholar] [CrossRef]
- Nabimeybodi, R.; Meyari, A.; Vahiddastjerdi, M.; Hajimehdipoor, H.; Ghasemi, E.; Bioos, S. The effect of flixweed-honey vaginal product on cervicitis: A clinical trial. Res. J. Pharmacogn. 2018, 5, 41–49. [Google Scholar]
- Banaeian, S.; Sereshti, M.; Rafieian, M.; Farahbod, F.; Kheiri, S. Comparison of vaginal ointment of honey and clotrimazole for treatment of vulvovaginal candidiasis: A random clinical trial. J. Mycol. Med. 2017, 27, 494–500. [Google Scholar] [CrossRef]
- Lavaf, M.; Simbar, M.; Mojab, F.; Alavi Majd, H.; Samimi, M. Comparison of honey and phenytoin (PHT) cream effects on intensity of pain and episiotomy wound healing in nulliparous women. J. Complement. Integr. Med. 2017, 15, 20160139. [Google Scholar] [CrossRef]
- Qayyum, S.; Sultana, A.; Heyat, M.B.B.; Rahman, K.; Akhtar, F.; Haq, A.U.; Alkhamis, B.A.; Alqahtani, M.A.; Gahtani, R.M. Therapeutic Efficacy of a Formulation Prepared with Linum usitatissimum L., Plantago ovata Forssk., and Honey on Uncomplicated Pelvic Inflammatory Disease Analyzed with Machine Learning Techniques. Pharmaceutics 2023, 15, 643. [Google Scholar] [CrossRef]
- Amiri Farahani, Ë.L.; Hasanpoor-Azghdy, S.B.; Kasraei, H.; Heidari, T. Comparison of the effect of honey and mefenamic acid on the severity of pain in women with primary dysmenorrhea. Arch. Gynecol. Obstet. 2017, 296, 277–283. [Google Scholar] [CrossRef]
- Yusuf, W.N.W.; Mohammad, W.M.Z.W.; Gan, S.H.; Mustafa, M.; Abd Aziz, C.B.; Sulaiman, S.A. Tualang honey ameliorates viral load, CD4 counts and improves quality of life in asymptomatic human immunodeficiency virus infected patients. J. Tradit. Complement. Med. 2019, 9, 249–256. [Google Scholar] [CrossRef]
- Semprini, A.; Braithwaite, I.; Corin, A.; Sheahan, D.; Tofield, C.; Helm, C.; Montgomery, B.; Fingleton, J.; Weatherall, M.; Beasley, R. Randomised controlled trial of topical kanuka honey for the treatment of acne. BMJ Open 2016, 6, e009448. [Google Scholar] [CrossRef] [PubMed]
- Alangari, A.A.; Morris, K.; Lwaleed, B.A.; Lau, L.; Jones, K.; Cooper, R.; Jenkins, R. Honey is potentially effective in the treatment of atopic dermatitis: Clinical and mechanistic studies. Immun. Inflamm. Dis. 2017, 5, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Jaffary, F.; Moradi, S.; Derakhshan, R.; Haftbaradaran, E. Effect of topical honey application along with intralesional injection of glucantime in the treatment of cutaneous leishmaniasis. BMC Complement. Altern. Med. 2007, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, I.; Hunt, A.; Riley, J.; Fingleton, J.; Kocks, J.; Corin, A.; Helm, C.; Sheahan, D.; Tofield, C.; Montgomery, B.; et al. Randomised controlled trial of topical kanuka honey for the treatment of rosacea. BMJ Open 2015, 5, e007651. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.S. Topical application of natural honey, beeswax and olive oil mixture for atopic dermatitis or psoriasis: Partially controlled, single-blinded study. Complement. Ther. Med. 2003, 11, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Haffejee, I.E.; Moosa, A. Honey in the treatment of infantile gastroenteritis. Br. Med. J. Clin. Res. Ed. 1985, 290, 1866–1867. [Google Scholar] [CrossRef]
- Nishimura, T.; Muta, H.; Hosaka, T.; Ueda, M.; Kishida, K.; Honey and Coughs Study Group of the Society of Ambulatory and General Paediatrics of Japan. Multicentre, randomised study found that honey had no pharmacological effect on nocturnal coughs and sleep quality at 1–5 years of age. Acta Paediatr. 2022, 111, 2157–2164. [Google Scholar] [CrossRef]
- Fakhr-Movahedi, A.; Mirmohammadkhani, M.; Ramezani, H. Effect of milk-honey mixture on the sleep quality of coronary patients: A clinical trial study. Clin. Nutr. ESPEN 2018, 28, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.M.; Beiler, J.; McMonagle, A.; Shaffer, M.L.; Duda, L.; Berlin, C.M., Jr. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch. Pediatr. Adolesc. Med. 2007, 161, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.A.; Rozen, J.; Kristal, H.; Laks, Y.; Berkovitch, M.; Uziel, Y.; Kozer, E.; Pomeranz, A.; Efrat, H. Effect of honey on nocturnal cough and sleep quality: A double-blind, randomized, placebo-controlled study. Pediatrics 2012, 130, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Shadkam, M.N.; Mozaffari-Khosravi, H.; Mozayan, M.R. A comparison of the effect of honey, dextromethorphan, and diphenhydramine on nightly cough and sleep quality in children and their parents. J. Altern. Complement. Med. 2010, 16, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Ayazi, P.; Mahyar, A.; Yousef-Zanjani, M.; Allami, A.; Esmailzadehha, N.; Beyhaghi, T. Comparison of the Effect of Two Kinds of Iranian Honey and Diphenhydramine on Nocturnal Cough and the Sleep Quality in Coughing Children and Their Parents. PLoS ONE 2017, 12, e0170277. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Albietz, J.M.; Tran, H.; Du Toit, C.; Li, A.H.; Yun, T.; Han, J.; Schmid, K.L. Treatment of contact lens related dry eye with antibacterial honey. Cont. Lens Anterior Eye 2017, 40, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Jia, T.; Liao, R.; Stapleton, F. Effect of a formulated eye drop with Leptospermum spp. honey on tear film properties. Br. J. Ophthalmol. 2020, 104, 1373–1377. [Google Scholar] [CrossRef]
- Albietz, J.M.; Schmid, K.L. Randomised controlled trial of topical antibacterial Manuka (Leptospermum species) honey for evaporative dry eye due to meibomian gland dysfunction. Clin. Exp. Optom. 2017, 100, 603–615. [Google Scholar] [CrossRef]
- Cernak, M.; Majtanova, N.; Cernak, A.; Majtan, J. Honey prophylaxis reduces the risk of endophthalmitis during perioperative period of eye surgery. Phytother. Res. 2012, 26, 613–616. [Google Scholar] [CrossRef]
- Craig, J.P.; Cruzat, A.; Cheung, I.M.Y.; Watters, G.A.; Wang, M.T.M. Randomized masked trial of the clinical efficacy of MGO Manuka Honey microemulsion eye cream for the treatment of blepharitis. Ocul. Surf. 2020, 18, 170–177. [Google Scholar] [CrossRef]
- Othman, Z.; Shafin, N.; Zakaria, R.; Hussain, N.H.; Mohammad, W.M. Improvement in immediate memory after 16 weeks of tualang honey (Agro Mas) supplement in healthy postmenopausal women. Menopause 2012, 18, 1219–1224. [Google Scholar] [CrossRef]
- Yahaya, R.; Zahary, M.N.; Othman, Z.; Ismail, R.; Nik Him, N.A.S.; Abd Aziz, A.; Dahlan, R.; Jusoh, A.F. Tualang honey supplementation as cognitive enhancer in patients with schizophrenia. Heliyon 2020, 6, e03948. [Google Scholar] [CrossRef] [PubMed]
- Gamaleldin, M.; Abraham, I.; Meabed, M.; Elberry, A.; Abdelhalim, S.; Waggas, D.; Hussein, R. Comparative effectiveness of adding omega-3 and Manuka honey combination to conventional therapy in preventing and treating oxidative stress in pediatric β-thalassemia major–a randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6058–6070. [Google Scholar] [CrossRef]
- Yong, P.Y.A.; Yip, A.J.W.; Islam, F.; Hong, H.J.; Teh, Y.E.; Tham, C.L.; Tan, J.W. The anti-allergic potential of stingless bee honey from different botanical sources via modulation of mast cell degranulation. BMC Complement. Med. Ther. 2023, 23, 307. [Google Scholar] [CrossRef]
- Iftikhar, F.; Arshad, M.; Rasheed, F.; Amraiz, D.; Anwar, P.; Gulfraz, M. Effects of acacia honey on wound healing in various rat models. Phytother. Res. 2010, 24, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, E.; Cicconi, R.; Mattei, M.; Muzi, M.G.; Canini, A. Acacia Honey and Chrysin Reduce Proliferation of Melanoma Cells Through Alterations in Cell Cycle Progression. Int. J. Oncol. 2010, 37, 973–981. [Google Scholar] [CrossRef]
- Aliyu, M.; Odunola, O.A.; Farooq, A.D.; Rasheed, H.; Mesaik, A.M.; Choudhary, M.I.; Erukainure, O.L. Molecular Mechanism of Antiproliferation Potential of Acacia Honey on NCI-H460 Cell Line. Nutr. Cancer 2013, 65, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef]
- Watanabe, K.; Rahmasari, R.; Matsunaga, A.; Haruyama, T.; Kobayashi, N. Anti-influenza viral effects of honey in vitro: Potent high activity of manuka honey. Arch. Med. Res. 2014, 45, 359–365. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. The anti-cancer effects of Tualang honey in modulating breast carcinogenesis: An experimental animal study. BMC Complement. Altern. Med. 2017, 17, 208. [Google Scholar] [CrossRef]
- Morales, P.; Haza, A.I. Antiproliferative and apoptotic effects of spanish honeys. Pharmacogn. Mag. 2013, 9, 231. [Google Scholar] [CrossRef]
- Bucekova, M.; Bugarova, V.; Godocikova, J.; Majtan, J. Demanding new honey qualitative standard based on antibacterial activity. Foods 2020, 9, 1263. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Guthami, F.M.A.; Ramadan, M.F.A.; Gethami, A.F.M.A.; Craig, A.M.; El-Seedi, H.R.; Rodríguez, I.; Serrano, S. The Bioactive Value of Tamarix gallica Honey from Different Geographical Origins. Insects 2023, 14, 319. [Google Scholar] [CrossRef]
- Luccas, F.S.; Fernandes, E.A.D.N.; Mazola, Y.T.; Bacchi, M.A.; Sarriés, G.A. Optimization of sample preparation of Brazilian honeys for TQ-ICP-MS analysis. Talanta Open 2022, 5, 100117. [Google Scholar] [CrossRef]
- Baroni, M.V.; Podio, N.S.; Badini, R.G.; Inga, M.; Ostera, H.A.; Cagnoni, M.; Gautier, E.A.; García, P.P.; Hoogewerff, J.; Wunderlin, D.A. Linking soil, water, and honey composition to assess the geographical origin of Argentinean honey by multielemental and isotopic analyses. J. Agric. Food Chem. 2015, 63, 4638–4645. [Google Scholar] [CrossRef]
- Weilert, T.M.; Ray, C.L.; Gawenis, J.A.; Brockman, J.D. Neutron activation analysis and ICP-MS for provenance of honey collected from American Midwest region. J. Radioanal. Nucl. Chem. 2022, 331, 4971–4981. [Google Scholar] [CrossRef]
- Chen, H.; Fan, C.; Chang, Q.; Pang, G.; Hu, X.; Lu, M.; Wang, W. Chemometric determination of the botanical origin for Chinese honeys on the basis of mineral elements determined by ICP-MS. J. Agric. Food Chem 2014, 62, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Grainger, M.N.; Klaus, H.; Hewitt, N.; Gan, H.; French, A.D. Graphical Discrimination of New Zealand Honey from International Honey Using Elemental Analysis. Biol. Trace Elem. Res. 2023, 202, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Chudzinska, M.; Baralkiewicz, D. Application of ICP-MS method of determination of 15 elements in honey with chemometric approach for the verification of their authenticity. Food Chem. Toxicol. 2011, 49, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Sajtos, Z.; Herman, P.; Harangi, S.; Baranyai, E. Elemental analysis of Hungarian honey samples and bee products by MP-AES method. Microchem. J. 2019, 149, 103968. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, T.; Zhou, J.; Chen, L. Application of stable isotopic and elemental composition combined with random forest algorithm for the botanical classification of Chinese honey. J. Food Compos. Anal. 2022, 110, 104565. [Google Scholar] [CrossRef]



| No | Honey | Pathology | Results | Ref. |
|---|---|---|---|---|
| 1 | Manuka, Tualang | Atopic dermatitis | Faster healing of the skin lesion | [179,182] |
| 2 | Honey not specified | Pediatric Gastroenteritis | Reduced diarrheic stools and length of hospital stay | [183] |
| 3 | Manuka | Pediatric β-thalassemia major | Reduction of oxidative stress; Improved Hemoglobin; Reduced frequency of necessary blood transfusions | [197] |
| 4 | Buckwheat, Tualang, Clover, Multifloral | Metabolic activity | Reduces inflammation, antioxidant activity, improved metabolic and cardiovascular parameters | [83,85,86,88] |
| 5 | Tualang | Cognitive function | Improves immediate memory in healthy individuals and learning performance in schizophrenia patients | [195,196] |
| 6 | Clover, Thyme, Manuka | Oncology | Improves chemo-radiotherapy-induced mucositis; Relieves pain; Improves hematologic parameters | [110,112,119,123] |
| 7 | Manuka | Ophthalmology | Improves dry eye symptoms; Accelerates healing in blepharitis | [190,191,192,193] |
| 8 | Apis mellifera, Manuka | Oral Health | Reduces bacterial plaque; Anti-inflammatory and soothing effect after dental procedures | [142,147,148] |
| 9 | Acacia, Manuka, Thyme | Upper respiratory tract diseases | Improves rhinosinusitis symptoms, ameliorates acute cough in children, reduces nasal bacterial colonization | [127,128,129,137] |
| 10 | Buckwheat, Eucalyptus, Labiatae, Citrus | Sleep Quality | Improves sleep quality in coronary patients and children | [185,186,187] |
| 11 | Tualang, Silk-cotton, Thyme, Multifloral | Surgery-related symptoms | Reduces post-operative pain, accelerates wound healing | [154,156,158,159,161] |
| 12 | Astragalus | Female urogenital pathology | Alleviates dysmenorrhea pain, reduces inflammation | [176] |
| 13 | Beri, Wildflower, Manuka, Multifloral, Tualang | Wound care | Improved pain score, faster healing of surgical wounds, pressure ulcers, diabetic foot ulcers | [47,48,58,60,61,69,70] |
| No | Authentication | Analytical Method | Aim | Ref. |
|---|---|---|---|---|
| 1 | botanical/geographical | ICP-MS and IRMS | differentiation among 7 varietal honey types originating in both Romania and France by means of elemental and isotopic profiling and emerging REEs | [15] |
| 2 | geographical | ICP-MS | differentiating the honeydew honey produced in three regions in Brazil by 39 elements (including REEs) | [35] |
| 3 | botanical/geographical | ICP-MS | discriminating between 18 monofloral and multifloral imported honey samples and 12 multifloral Jordanian local samples (elemental profiling including REEs) | [36] |
| 4 | botanical/soil distribution | ICP-MS and ICP-OES | determining the traceability of the honey chain with regard to the honey, flowers, and soil origin by comparing the lanthanide distributions of 17 different monofloral honeys | [39] |
| 5 | geographical | ICP-MS | analyzing 40 metals (including REEs) in multifloral honey samples from Tanzania, Italia, Kazakhstan, the Balkans, and South America | [38] |
| 6 | botanical | ICP-MS | evaluating the trace elements and REE content in four monofloral honeys | [37] |
| 7 | botanical/geographical | ICP-MS | elemental metabolomic determination for honey differentiation according to the geographical (Greek vs Poland) and botanic origin | [29] |
| 8 | botanical/geographical/entomological origin of bees | TQ-ICP-MS | determination of the best sample digestion procedure for elemental analysis of Brazilian honeys from two species. Authentication based on elemental and composition profile. | [208] |
| 9 | geographical | ICP-MS, IRMS and TIMS | fingerprint determination to link soil, water, and honey composition in order to assess the geographical origin of Argentinean honey | [209] |
| 10 | geographical | ICP-MS and INAA | demonstrating that elemental analysis may be useful in differentiating honey sourced from Montana against honey from North and South Dakota | [210] |
| 11 | botanical | ICP-MS | measuring the concentration of trace and toxic elements in four unifloral honeys produced in Sardinia | [31] |
| 12 | botanical | ICP-MS | classifying Chinese honeys according to their botanical origin by the mineral element content and chemometric methods | [211] |
| 13 | geographical | ICP-MS | utilizing the elemental fingerprinting of honey to differentiate New Zealand (NZ) honey from honey of international origin (34 other countries) | [212] |
| 14 | botanical | ICP-MS | differentiating the mineral content of 55 honey samples originating from three types (honeydew, buckwheat, and rape honey) from different areas in Poland | [213] |
| 15 | botanical | ICP-OES and IRMS | investigating the impact of botanical origin on the stable isotopes and elemental content of Greek unifloral honeys | [30] |
| 16 | botanical/geographical | MP-AES | differentiation of the geological origin of honey samples from Central Hungary and Western Transdanubia as well as differentiation among five honey types | [214] |
| 17 | botanical/geographical | ICP-MS and IRMS | verifying the relationship between stable isotope ratios together with the elemental content and the geographical and botanical origin of honey | [31] |
| 18 | botanical | ICP-MS and IRMS | determination of isotope ratios and element chemical fingerprints in order to distinguish between six Chinese honeys | [215] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdas, T.M.; David, M.; Hategan, A.R.; Filip, G.A.; Magdas, D.A. Geographical Origin Authentication—A Mandatory Step in the Efficient Involvement of Honey in Medical Treatment. Foods 2024, 13, 532. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13040532
Magdas TM, David M, Hategan AR, Filip GA, Magdas DA. Geographical Origin Authentication—A Mandatory Step in the Efficient Involvement of Honey in Medical Treatment. Foods. 2024; 13(4):532. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13040532
Chicago/Turabian StyleMagdas, Tudor Mihai, Maria David, Ariana Raluca Hategan, Gabriela Adriana Filip, and Dana Alina Magdas. 2024. "Geographical Origin Authentication—A Mandatory Step in the Efficient Involvement of Honey in Medical Treatment" Foods 13, no. 4: 532. https://0-doi-org.brum.beds.ac.uk/10.3390/foods13040532