Antimicrobial Properties of Encapsulated Antimicrobial Natural Plant Products for Ready-to-Eat Carrots
Abstract
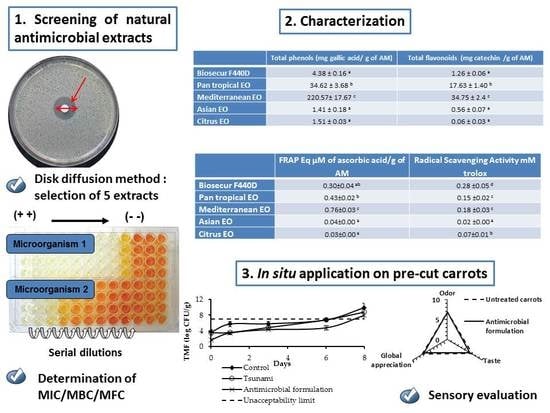
:1. Introduction
2. Materials and Methods
2.1. Antimicrobial Extracts
2.2. Preparation of Bacterial Cultures
2.3. Preliminary Study
2.4. Antimicrobial Efficiency
2.5. Total Phenol Determination
2.6. Radical Scavenging Activity (DPPH)
2.7. Ferric-Reducing Antioxidant Power (FRAP)
2.8. Determination of Total Flavonoids Content
2.9. In Situ Test on Pre-Cut Carrots
2.9.1. Antimicrobial Loaded Emulsion
2.9.2. Samples Preparation
2.9.3. Shelf-life Estimation
2.10. Sensory Evaluation
2.11. Statistical Analysis
3. Results
3.1. Preliminary Study
3.2. Determination of MIC, MBC and MFC
3.3. Total Phenols and Flavonoids
3.4. Radical Scavenging Activity and FRAP
3.5. In Situ Analysis
3.6. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rai, M. Natural Antimicrobials in Food Safety and Quality; CABI: Oxfordshire, UK, 2011. [Google Scholar]
- Gagnaire, F.; Marignac, B.; Hecht, G.; Héry, M. Sensory irritation of acetic acid, hydrogen peroxide, peroxyacetic acid and their mixture in mice. Ann. Occup. Hyg. 2002, 46, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Khaneghah, A.M.; de Souza Sant’Ana, A. Essential Oils in Food Processing: Chemistry, Safety and Applications; John Wiley & Sons: West Sussex, UK, 2017. [Google Scholar]
- Nollet, L.M.; Rathore, H.S. Green Pesticides Handbook: Essential Oils for Pest Control; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lee, Y.-L.; Cesario, T.; Wang, Y.; Shanbrom, E.; Thrupp, L. Antibacterial activity of vegetables and juices. Nutrition 2003, 19, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.R.; Chidambara Murthy, K.; Jayaprakasha, G.; Chetti, M.B.; Patil, B.S. Bioactive compounds from Mexican lime (Citrus aurantifolia) juice induce apoptosis in human pancreatic cells. J. Agric. Food Chem. 2009, 57, 10933–10942. [Google Scholar] [CrossRef] [PubMed]
- Ben-Fadhel, Y.; Leroy, V.; Dussault, D.; St-Yves, F.; Lauzon, M.; Salmieri, S.; Jamshidian, M.; Vu, D.K.; Lacroix, M. Combined effects of marinating and γ-irradiation in ensuring safety, protection of nutritional value and increase in shelf-life of ready-to-cook meat for immunocompromised patients. Meat Sci. 2016, 118, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of Natural Antimicrobials for Food Preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoli, A.; Cirak, C.; Silva, J. Hypericum species as sources of valuable essential oils. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 29–47. [Google Scholar]
- Ayari, S.; Dussault, D.; Millette, M.; Hamdi, M.; Lacroix, M. Changes in membrane fatty acids and murein composition of Bacillus cereus and Salmonella Typhi induced by gamma irradiation treatment. Int. J. Food Microbiol. 2009, 135, 1–6. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety-E-Book: A Guide for Health Care Professionals; Elsevier Health Sciences: London, UK, 2013. [Google Scholar]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Santos, M.; Martins, S.; Veríssimo, C.; Nunes, M.; Lima, A.; Ferreira, R.; Pedroso, L.; Sousa, I.; Ferreira, M. Essential oils as antibacterial agents against food-borne pathogens: Are they really as useful as they are claimed to be? J. Food Sci. Technol. 2017, 54, 4344–4352. [Google Scholar] [CrossRef]
- Sivapalasingam, S.; Friedman, C.R.; Cohen, L.; Tauxe, R.V. Fresh Produce: A Growing Cause of Outbreaks of Foodborne Illness in the United States, 1973 through 1997. J. Food Prot. 2004, 67, 2342–2353. [Google Scholar] [CrossRef]
- Jalava, K.; Hakkinen, M.; Valkonen, M.; Nakari, U.-M.; Palo, T.; Hallanvuo, S.; Ollgren, J.; Siitonen, A.; Nuorti, J.P. An Outbreak of Gastrointestinal Illness and Erythema Nodosum from Grated Carrots Contaminated with Yersinia pseudotuberculosis. J. Infect. Dis. 2006, 194, 1209–1216. [Google Scholar] [CrossRef]
- Gaynor, K.; Park, S.; Kanenaka, R.; Colindres, R.; Mintz, E.; Ram, P.; Kitsutani, P.; Nakata, M.; Wedel, S.; Boxrud, D. International foodborne outbreak of Shigella sonnei infection in airline passengers. Epidemiol. Infect. 2009, 137, 335–341. [Google Scholar] [CrossRef]
- Da Silva Felício, M.T.; Hald, T.; Liebana, E.; Allende, A.; Hugas, M.; Nguyen-The, C.; Johannessen, G.S.; Niskanen, T.; Uyttendaele, M.; McLauchlin, J. Risk ranking of pathogens in ready-to-eat unprocessed foods of non-animal origin (FoNAO) in the EU: Initial evaluation using outbreak data (2007–2011). Int. J. Food Microbiol. 2015, 195, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cruz, S.; Acedo-Félix, E.; Díaz-Cinco, M.; Islas-Osuna, M.A.; González-Aguilar, G.A. Efficacy of sanitizers in reducing Escherichia coli O157:H7, Salmonella spp. and Listeria monocytogenes populations on fresh-cut carrots. Food Control 2007, 18, 1383–1390. [Google Scholar] [CrossRef]
- Banwart, G. Basic Food Microbiology; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Tournas, V. Spoilage of vegetable crops by bacteria and fungi and related health hazards. Crit. Rev. Microbiol. 2005, 31, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.; Hutchison, M. Ineffective hand washing and the contamination of carrots after using a field latrine. Lett. Appl. Microbiol. 2016, 62, 299–303. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Saltaji, S.; Khlifi, M.A.; Salmieri, S.; Vu, K.D.; Lacroix, M. Active edible coating and γ-irradiation as cold combined treatments to assure the safety of broccoli florets (Brassica oleracea L.). Int. J. Food Microbiol. 2017, 241, 30–38. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.I.; Angioni, A.; Dessi, S.; Marzouki, N.; Cabras, P. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J. Agric. Food Chem. 2007, 55, 963–969. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Takala, P.N.; Vu, K.D.; Salmieri, S.; Khan, R.A.; Lacroix, M. Antibacterial effect of biodegradable active packaging on the growth of Escherichia coli, Salmonella typhimurium and Listeria monocytogenes in fresh broccoli stored at 4 °C. LWT-Food Sci. Technol. 2013, 53, 499–506. [Google Scholar] [CrossRef]
- Babic, I.; Hilbert, G.; Nguyen-The, C.; Guiraud, J. The yeast flora of stored ready-to-use carrots and their role in spoilage. Int. J. Food Sci. Technol. 1992, 27, 473–484. [Google Scholar] [CrossRef]
- Itohan, A.M.; Peters, O.; Kolo, I. Bacterial contaminants of salad vegetables in abuja municipal area council, Nigeria. Malays. J. Microbiol. 2011, 7, 111–114. [Google Scholar]
- MAPAQ. Lignes Directrices et Normes Pour L’interprétation des Résultats Analytiques en Microbiologie Alimentaire. Available online: https://www.mapaq.gouv.qc.ca/fr/Publications/recueil.pdf (accessed on 31 October 2019).
- da Silva, A.C.; de Freitas Santos, P.D.; Palazzi, N.C.; Leimann, F.V.; Fuchs, R.H.B.; Bracht, L.; Gonçalves, O.H. Production and characterization of curcumin microcrystals and evaluation of the antimicrobial and sensory aspects in minimally processed carrots. Food Funct. 2017, 8, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Ghabraie, M.; Vu, K.D.; Tata, L.; Salmieri, S.; Lacroix, M. Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT-Food Sci. Technol. 2016, 66, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Lopez, P.; Sanchez, C.; Batlle, R.; Nerin, C. Solid-and vapor-phase antimicrobial activities of six essential oils: Susceptibility of selected foodborne bacterial and fungal strains. J. Agric. Food Chem. 2005, 53, 6939–6946. [Google Scholar] [CrossRef]
- Dussault, D.; Vu, K.D.; Lacroix, M. In vitro evaluation of antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Wogiatzi, E.; Gougoulias, N.; Papachatzis, A.; Vagelas, I.; Chouliaras, N. Greek Oregano Essential Oils Production, Phytotoxicity and Antifungal Activity. Biotechnol. Biotechnol. Equip. 2009, 23, 1150–1152. [Google Scholar] [CrossRef] [Green Version]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Janitha, P.; Wanasundara, P. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Jirovetz, L.; Buchbauer, G.; Eller, G.A.; Stoilova, I.; Krastanov, A.; Stoyanova, A.; Geissler, M. Composition and antioxidant activities of the essential oil of cinnamon (Cinnamomum zeylanicum Blume) leaves from Sri Lanka. J. Essent. Oil Bear. Plants 2006, 9, 170–182. [Google Scholar] [CrossRef]
- Bang, K.-H.; Lee, D.-W.; Park, H.-M.; Rhee, Y.-H. Inhibition of fungal cell wall synthesizing enzymes by trans-cinnamaldehyde. Biosci. Biotechnol. Biochem. 2000, 64, 1061–1063. [Google Scholar] [CrossRef]
- Silva, C.D.B.D.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.; Deans, S. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.; Yang, H.; Yuan, Y.; Yue, T. Antifungal mechanism of cinnamaldehyde and citral combination against Penicillium expansum based on FT-IR fingerprint, plasma membrane, oxidative stress and volatile profile. RSC Adv. 2018, 8, 5806–5815. [Google Scholar] [CrossRef]
- Nakahara, K.; Alzoreky, N.S.; Yoshihashi, T.; Nguyen, H.T.; Trakoontivakorn, G. Chemical composition and antifungal activity of essential oil from Cymbopogon nardus (citronella grass). Jpn. Agric. Res. Q. JARQ 2013, 37, 249–252. [Google Scholar] [CrossRef]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef]
- Aguiar, R.W.D.S.; Ootani, M.A.; Ascencio, S.D.; Ferreira, T.P.; Santos, M.M.d.; Santos, G.R.d. Fumigant antifungal activity of Corymbia citriodora and Cymbopogon nardus essential oils and citronellal against three fungal species. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Pereira, F.D.O.; Wanderley, P.A.; Viana, F.A.C.; Lima, R.B.D.; Sousa, F.B.D.; Lima, E.D.O. Growth inhibition and morphological alterations of Trichophyton rubrum induced by essential oil from Cymbopogon winterianus Jowitt ex Bor. Braz. J. Microbiol. 2011, 42, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simic, A.; Rančic, A.; Sokovic, M.; Ristic, M.; Grujic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Essential oil composition of Cymbopogon winterianus. and Carum carvi. and their antimicrobial activities. Pharm. Biol. 2008, 46, 437–441. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Carvajal, A.; Arguello, H.; Martínez-Lobo, F.; Naharro, G.; Rubio, P. Antibacterial activity and mode of action of a commercial citrus fruit extract. J. Appl. Microbiol. 2013, 115, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabro, M.; Galtieri, V.; Cutroneo, P.; Tommasini, S.; Ficarra, P.; Ficarra, R. Study of the extraction procedure by experimental design and validation of a LC method for determination of flavonoids in Citrus bergamia juice. J. Pharm. Biomed. Anal. 2004, 35, 349–363. [Google Scholar] [CrossRef]
- Inoue, Y.; Hoshino, M.; Takahashi, H.; Noguchi, T.; Murata, T.; Kanzaki, Y.; Hamashima, H.; Sasatsu, M. Bactericidal activity of Ag–zeolite mediated by reactive oxygen species under aerated conditions. J. Inorg. Biochem. 2002, 92, 37–42. [Google Scholar] [CrossRef]
- Amanatidou, A.; Slump, R.A.; Gorris, L.G.M.; Smid, E.J. High Oxygen and High Carbon Dioxide Modified Atmospheres for Shelf-life Extension of Minimally Processed Carrots. J. Food Sci. 2000, 65, 61–66. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989. [Google Scholar] [CrossRef]


| Common Name | Botanic Name | Part | Compositions (%) * |
|---|---|---|---|
| Bergamote EO | Citrus bergamia | Zest | Limonene (36.2), Linalyle acetate (29.7), linalool (13.2), γ-terpinene (6.8), β-pinene (5.4) |
| Pan tropical EO | Cinnamomum verrum | Peel | E-cinnamaldehyde (55.1), cinnamyl acetate (9.6), β-caryophyllene (4.0) |
| Citrus EO | Cymbopogon winterianus | Aerial part | Citronellal (35.4), geraniol (20.1), Citronellol (12.2), elemol (4.6), Limonene (3.0), citronellyl acetate (2.9), germacrene D (2.7), geranyl acetate de (2.5), linalool (0.6) |
| Ginger EO | Zingiber officinalis | Rhizome | α-zingiberene (25.4), β-sesquiphellandrene + α-curcumene (13.9), Camphene (10.5), β-phellandrene + 1, 8-cineole (8.3), β-bisabolene + β-selinene (7.7), E,E-α-farnesene (4.2), α-pinene (3.3) |
| Asian EO | Cymbopogon flexuosus | Herb | Geranial (39.1), neral (31.6), geraniol (6.7), geranyl acetate (3.7) |
| Marjolaine shells EO | Origanum majorana | Flower top | Terpinene-4-ol (28.0), γ-terpinene (15.5), α-terpinene (9.5), Cis-thuyanol (7.3), α-terpineol (3.7) |
| Peppermint EO | Mentha x piperita | Aerial part | Menthol (30.6), menthone (29.3), 1,8-cineole + β-phellandrene (5.2), menthyl acetate (4.5), neomenthol (3.1), Isomenthone (4.4), menthofurane (4.2), Limonene (2.4) |
| Myrte cineole EO | Myrtus communis | leaf | α-pinene (51.5), 1,8-cineole (23.9), Limonene (10.4), Linalool (3.0) |
| Sweet orange EO | Citrus sinensis | Zest | Limonene (94.8) |
| Tea tree EO | Melaleuca alternifolia | Leaf | Terpinene-4-ol (37.6), γ-terpinene (21.1), α-terpinene (10.1), Terpinolene (4.8), 1,8-cineole + β-phellandrene (4.2), α-pinene (2.6), α-terpineol (2.5) |
| Mediterranean EO | Origanum compactum | Flower top | Carvacrol (46.1), thymol (17.6), γ-terpinene+ trans-β-ocimene (14.8), p-cymene (8.5) |
| Thyme leaf savory EO | Thymus satureioides | Flower top | Borneol (27.0), α-terpineol (11.9), camphene (10.5), α-pinene + α-thuyene (6.5), β-caryophyllene (5.5), Carvacrol (5.3), p-cymene (3.9), Linalol (3.7), Terpinene-4-ol + methyl carvacrol ether (2.9), 1,8-cineole + β-phellandrene (2.9), Thymol (2.8) |
| Cloves EO | Eugenia caryophyllus | Floral button | Eugenol (81.8), Eugenyl acetate (12.9), β-caryophyllene (3.4) |
| Thyme thymol EO | Thymus vulgaris CT6 | Flower top | Thymol (46.6), p-cymene (16.9), γ-terpinene (9.3), Linalool (4.1), Carvacrol (3.5) |
| Inhibition Diameter: Mean ± std.dev (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram Positive | Gram Negative | Yeast | Molds | |||||||
| L. monocytogenes | B. subtilis | E. faecium | S. aureus | S. Typhimurium | E. coli | C. albicans | A. flavus | P. chrysogenum | ||
| 1 | Biosecur F440D | 16.6 ± 1.9 | 18.9 ± 1.0 | 12.3 ± 0.7 | 25.4 ± 2.4 | 12.5 ± 1.1 | 13.7 ± 0.9 | 22.8 ± 1.6 | 14.1 ± 0.7 | 13.6 ± 3.0 |
| 2 | Cranberry juice | 8.7 ± 0.9 | 9.3 ± 1.6 | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 | 7.0 ± 1.2 | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 |
| 3 | Bergamote EO | 6.0 ± 0.0 | 13.7 ± 1.2 | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 | 10.7 ± 0.4 | 6.0 ± 0.0 | 8.5 ± 0.3 |
| 4 | Citrus EO * | 8.4 ± 0.6 | 68.7 ± 4.9 | 13.9 ± 0.4 | 35.6 ± 2.8 | 13.8 ± 1.0 | 14.8 ± 2.0 | 36.2 ± 5.5 | 22.4 ± 5.2 | 45.7 ± 4.0 |
| 5 | Cloves EO | 14.8 ± 1.2 | 24.9 ± 3.4 | 19.0 ± 1.9 | 22.0 ± 2.5 | 20.0 ± 0.8 | 20.1 ± 3.3 | 27.2 ± 0.7 | 38.6 ± 1.2 | 41.7 ± 1.2 |
| 6 | Marjoram EO | 13.9 ± 0.9 | 17.4 ± 3.5 | 16.1 ± 0.7 | 17.1 ± 0.8 | 17.3 ± 1.1 | 19.1 ± 2.4 | 13.0 ± 0.2 | 6.0 ± 0.0 | 11.2 ± 0.4 |
| 7 | Pepper menthe EO | 7.8 ± 0.4 | 19.1 ± 3.4 | 15.5 ± 0.9 | 18.9 ± 3.3 | 13.3 ± 0.4 | 14.4 ± 1.9 | 31.3 ± 1.8 | 6.0 ± 0.0 | 9.9 ± 1.1 |
| 8 | Sweet orange EO | 6.0 ± 0.0 | 14.0 ± 1.5 | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 | 11.1 ± 0.9 | 6.0 ± 0.0 | 8.5 ± 0.1 |
| 9 | Mediterranean EO | 23.8 ± 0.5 | 44.3 ± 4.1 | 33.9 ± 4.4 | 42.7 ± 4.0 | 28.5 ± 3.6 | 27.2 ± 2.5 | 52.0 ± 1.6 | 59.0 ± 2.6 | 80.0 ± 0.0 |
| 10 | Tea tree EO | 12.2 ± 0.4 | 17.2 ± 1.6 | 16.5 ± 0.8 | 18.3 ± 3.9 | 16.7 ± 2.2 | 17.3 ± 1.7 | 12.3 ± 1.3 | 6.0 ± 0.0 | 9.5 ± 0.4 |
| 11 | Thyme savory leaves EO | 11.3 ± 0.3 | 21.3 ± 3.7 | 16.0 ± 0.9 | 27.6 ± 2.6 | 17.7 ± 1.5 | 19.3±3.6 | 30.6 ± 1.9 | 20.5 ± 1.9 | 33.4 ± 0.5 |
| 12 | Myrte EO | 8.6 ± 0.4 | 11.0 ± 1.7 | 6.8 ± 0.9 | 9.3 ± 0.9 | 10.1 ± 2.4 | 8.7 ± 0.6 | 12.8 ± 1.2 | 6.0 ± 0.0 | 11.3 ± 1.3 |
| 13 | Ginger EO | 6.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 | 7.8 ± 2.9 | 6.0 ± 0.0 | 7.1 ± 1.3 | 12.4 ± 0.6 | 16.3 ± 1.4 | 11.4 ± 0.4 |
| 14 | Pan tropical EO | 31.1 ± 3.4 | 30.6 ± 2.1 | 23.7 ± 0.3 | 25.4 ± 2.1 | 32.0 ± 6.4 | 29.2 ± 1.3 | 61.0 ± 5.8 | 70.3 ± 3.4 | 63.0 ± 0.2 |
| 15 | Citral EO | 12.5 ± 1.4 | 10.2 ± 1.3 | 11.8 ± 1.6 | 18.4 ± 0.8 | 11.5 ± 1.4 | 10.4 ± 0.6 | 80.0 ± 0.0 | 23.0 ± 3.0 | 80.0 ± 0.0 |
| 16 | Asian EO | 8.8 ± 0.3 | 10.3 ± 2.6 | 9.2 ± 0.6 | 22.5 ± 1.2 | 9.6 ± 1.0 | 10.2 ± 0.7 | 42.7 ± 1.6 | 62.6 ± 6.1 | 80.0 ± 0.0 |
| 17 | Thyme thymol EO | 32.1 ± 2.2 | 41.4 ± 4.0 | 26.9 ± 3.4 | 31.3 ± 4.0 | 27.2 ± 3.7 | 30.5 ± 4.0 | 53.9 ± 2.6 | 38.3 ± 2.3 | 44.2 ± 5.3 |
| MIC, MBC and MFC Expressed in parts-per-million, PPM (Mean Value ± SD, n =3) | |||||||
|---|---|---|---|---|---|---|---|
| Biosecur F440D | Pan Tropical EO | Mediterranean EO | Asian EO | Citrus EO | Tween 80 2.5% | ||
| L. monocytogenes | MIC | 171 ± 5 | 621 ± 3 | 619 ± 2 | 4974 ± 0 | 4974 ± 0 | > 12500 |
| MBC | 171 ± 0 | 1241 ± 5 | 1237 ± 3 | 4974 ± 0 | 4974 ± 0 | - | |
| B. subtilis | MIC | 33 ± 1 | 1241 ± 6 | 1237 ± 3 | 2487 ± 0 | 4974 ± 0 | > 12500 |
| MBC | 33 ± 0 | 1241 ± 0 | 2470 ± 0 | 4974 ± 0 | 4974 ± 0 | - | |
| E. faecium | MIC | 142 ± 33 | 1241 ± 6 | 2474 ± 7 | 4979 ± 0 | 4974 ± 0 | > 12500 |
| MBC | 142 ± 28 | 2488 ± 8 | 4947 ± 10 | 4979 ± 7 | 4974 ± 0 | - | |
| S. aureus | MIC | 17 ± 0 | 1050 ± 0 | 1049 ± 1 | 1056 ± 0 | 2474 ± 0 | > 12500 |
| MBC | 17 ± 0 | 2227 ± 0 | 2224 ± 0 | 2239 ± 0 | 2474 ± 0 | - | |
| S. Typhimurium | MIC | 171 ± 5 | 621 ± 3 | 309 ± 1 | 1245 ± 2 | 4974 ± 0 | > 12500 |
| MBC | 171 ± 4 | 621 ± 2 | 619 ± 1 | 1245 ± 1 | 4974 ± 0 | - | |
| E. coli | MIC | 114 ± 3 | 621 ± 3 | 619 ± 2 | 1245 ± 2 | 2474 ± 0 | > 12500 |
| MBC | 114 ± 2 | 1243 ± 5 | 619 ± 1 | 1245 ± 0 | 2474 ± 0 | - | |
| C. albicans | MIC | 427 ± 12 | 155 ± 1 | 155 ± 0 | 311 ± 0 | 1245 ± 0 | > 12500 |
| MFC | 628 ± 0 | 621 ± 3 | 618 ± 1 | 311 ± 0 | 1245 ± 0 | - | |
| A. flavus | MIC | 836 ± 23 | 621 ± 3 | 2474 ± 7 | 4979 ± 0 | 4979 ± 0 | > 12500 |
| MFC | 1261 ± 26 | 621 ± 1 | 4958 ± 5 | 4979 ± 7 | 4979 ± 0 | - | |
| P. chrysogenum | MIC | 552 ± 11 | 155 ± 1 | 1237 ± 3 | 622 ± 1 | 1245 ± 0 | > 12500 |
| MFC | 609 ± 76 | 155 ± 1 | 2477 ± 5 | 622 ± 0 | 1245 ± 0 | - | |
| Natural Antimicrobial Products | Total Phenols (mg gallic acid/g of AM) * | Total Flavonoids (mg catechin/g of AM) * |
|---|---|---|
| Biosecur F440D | 4.38 ± 0.16 a | 1.26 ± 0.06 a |
| Pan tropical EO | 34.62 ± 3.68 b | 17.63 ± 1.40 b |
| Mediterranean EO | 220.57 ± 17.67 c | 34.75 ± 2.4 c |
| Asian EO | 1.41 ± 0.18 a | 0.56 ± 0.07 a |
| Citrus EO | 1.51 ± 0.03 a | 0.06 ± 0.03 a |
| Natural Antimicrobial Products | FRAP * | Radical Scavenging Activity * | |
|---|---|---|---|
| Eq µM of Ascorbic acid/g of AM | mM Trolox | mM AA | |
| Biosecur F440D | 0.30 ± 0.04 ab | 0.28 ± 0.05 d | 0.29 ± 0.05 d |
| Pan tropical EO | 0.43 ± 0.02 b | 0.15 ± 0.02 c | 0.15 ± 0.02 c |
| Mediterranean EO | 0.76 ± 0.03 c | 0.18 ± 0.03 c | 0.19 ± 0.03 c |
| Asian EO | 0.04 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| Citrus EO | 0.03 ± 0.00 a | 0.07 ± 0.01 b | 0.07 ± 0.01 b |
| Sample | Growth Rate of TMF (Ln CFU/g/day) |
|---|---|
| Control | 0.2193 |
| Tsunami | 0.1852 |
| Antimicrobial formulation | 0.1291 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Fadhel, Y.; Maherani, B.; Aragones, M.; Lacroix, M. Antimicrobial Properties of Encapsulated Antimicrobial Natural Plant Products for Ready-to-Eat Carrots. Foods 2019, 8, 535. https://0-doi-org.brum.beds.ac.uk/10.3390/foods8110535
Ben-Fadhel Y, Maherani B, Aragones M, Lacroix M. Antimicrobial Properties of Encapsulated Antimicrobial Natural Plant Products for Ready-to-Eat Carrots. Foods. 2019; 8(11):535. https://0-doi-org.brum.beds.ac.uk/10.3390/foods8110535
Chicago/Turabian StyleBen-Fadhel, Yosra, Behnoush Maherani, Melinda Aragones, and Monique Lacroix. 2019. "Antimicrobial Properties of Encapsulated Antimicrobial Natural Plant Products for Ready-to-Eat Carrots" Foods 8, no. 11: 535. https://0-doi-org.brum.beds.ac.uk/10.3390/foods8110535