Land Snails as a Valuable Source of Fatty Acids: A Multivariate Statistical Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
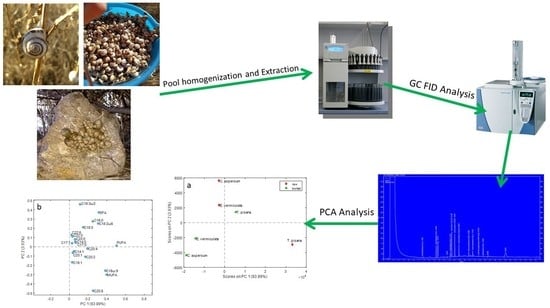
2.2. Sample Collection and Preparation
2.3. Extraction of Fatty Acids and Gas Chromatography with a Flame Ionization Detector (GC-FID) Analysis
2.4. Validation of the GC-FID Method
2.5. Data Collection and Statistical Analysis
3. Results
3.1. Fatty Acid Profiles
3.2. Fatty Acids of Raw Samples
3.3. Fatty Acids after Heat Treatment
3.4. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pellati, R. Lumache e Consumi. Available online: http://www.fosan.it/notiziario/31_lumache_e_consumi.html (accessed on 4 November 2019).
- Cicero, A.; Giangrosso, G.; Cammilleri, G.; Macaluso, A.; Currò, V.; Galuppo, L.; Vargetto, D.; Vicari, D.; Ferrantelli, V. Microbiological and Chemical Analysis of Land Snails Commercialised in Sicily. Ital. J. Food Saf. 2015, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Milinsk, M.C.; das Graças Padre, R.; Hayashi, C.; de Oliveira, C.C.; Visentainer, J.V.; de Souza, N.E.; Matsushita, M. Effects of feed protein and lipid contents on fatty acid profile of snail (Helix aspersa maxima) meat. J. Food Compos. Anal. 2006, 19, 212–216. [Google Scholar] [CrossRef]
- Alasalvar, C.; Pelvan, E.; Topal, B. Effects of roasting on oil and fatty acid composition of Turkish hazelnut varieties (Corylus avellana L.). Int. J. Food Sci. Nutr. 2010, 61, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Ayas, D.; Ozogul, Y.; Ozogul, İ.; Uçar, Y. The effects of season and sex on fat, fatty acids and protein contents of Sepia officinalis in the northeastern Mediterranean Sea. Int. J. Food Sci. Nutr. 2012, 63, 440–445. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Çiçek, E.; Polat, A.; Kuley, E. Fat content and fatty acid compositions of 34 marine water fish species from the Mediterranean Sea. Int. J. Food Sci. Nutr. 2009, 60, 464–475. [Google Scholar] [CrossRef]
- Tangolar, S.G.; Özoğul, Y.; Tangolar, S.; Torun, A. Evaluation of fatty acid profiles and mineral content of grape seed oil of some grape genotypes. Int. J. Food Sci. Nutr. 2009, 60, 32–39. [Google Scholar] [CrossRef]
- Tokuşoğlu, Ö. The quality properties and saturated and unsaturated fatty acid profiles of quail egg: The alterations of fatty acids with process effects. Int. J. Food Sci. Nutr. 2006, 57, 537–545. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Olgunoglu, A.I. Fatty acid profile and mineral content of the wild snail (Helix pomatia) from the region of the south of the Turkey. Eur. Food Res. Technol. 2005, 221, 547–549. [Google Scholar] [CrossRef]
- Szkucik, K.; Ziomek, M.; Paszkiewicz, W.; Drozd, Ł.; Gondek, M.; Knysz, P. Fatty acid profile in fat obtained from edible part of land snails harvested in Poland. J. Vet. Res. 2018, 62, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Purwaningsih, S.; Suseno, S.H.; Salamah, E.; Mulyaningtyas, J.R.; Dewi, Y.P. Effect of boiling and steaming on the profile fatty acids and cholesterol in muscle tissue of molluscs. Int. Food Res. J. 2015, 22, 1087–1094. [Google Scholar]
- Lazaridou-Dimitriadou, M.; Kattoulas, M.E. Energy flux in a natural population of the land snail Eobania vermiculata (Müller) (Gastropoda: Pulmonata: Stylommatophora) in Greece. Can. J. Zool. 1991, 69, 881–891. [Google Scholar] [CrossRef]
- Nicolai, A. The Impact of Diet Treatment on Reproduction and Thermophysiological Processes in the Land Snails Cornu Aspersum and Helix Pomatia. Ph.D. Thesis, Universität Bremen, Bremen, Germany, 2010. [Google Scholar]
- Odendaal, L.J.; Haupt, T.M.; Griffiths, C.L. The alien invasive land snail Theba pisana in the West Coast National Park: Is there cause for concern? Koedoe 2008, 50, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Bertino, E.; Giribaldi, M.; Baro, C.; Giancotti, V.; Pazzi, M.; Peila, C.; Tonetto, P.; Arslanoglu, S.; Moro, G.E.; Cavallarin, L.; et al. Effect of prolonged refrigeration on the lipid profile, lipase activity, and oxidative status of human milk. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in quality in the analytical laboratory. I. Traceability and measurement uncertainty of analytical results. TrAC Trends Anal. Chem. 2004, 23, 480–490. [Google Scholar] [CrossRef]
- Pantano, L.; Cascio, G.L.; Alongi, A.; Cammilleri, G.; Vella, A.; Macaluso, A.; Cicero, N.; Migliazzo, A.; Ferrantelli, V. Fatty acids determination in Bronte pistachios by gas chromatographic method. Nat. Prod. Res. 2016, 30, 2378–2382. [Google Scholar] [CrossRef]
- Raimondi, S.; Luciani, R.; Sirangelo, T.M.; Amaretti, A.; Leonardi, A.; Ulrici, A.; Foca, G.; D’Auria, G.; Moya, A.; Zuliani, V.; et al. Microbiota of sliced cooked ham packaged in modified atmosphere throughout the shelf life: Microbiota of sliced cooked ham in MAP. Int. J. Food Microbiol. 2019, 289, 200–208. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Ekin, İ. A comparative study on fatty acid content of main organs and lipid classes of land snails Assyriella escheriana and Assyriella guttata distributed in southeastern Anatolia. Ital. J. Food Sci. 2015, 27, 75–81. [Google Scholar]
- Baker, G.H. The Biology and Control of White Snails (Mollusca: Helicidae), Introduced Pests in Australia; Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1986. [Google Scholar]
- Baker, G.H. Damage, Population Dynamics, Movement and Control of Pest Helicid Snails in Southern Australia; Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1989; pp. 175–185. [Google Scholar]
- Cowie, R.H. The Life-Cycle and Productivity of the Land Snail Theba pisana (Mollusca: Helicidae). J. Anim. Ecol. 1984, 53, 311–325. [Google Scholar] [CrossRef]
- Chevalier, L.; Coz, M.; Charrier, M. Influence of inorganic compounds on food selection by the brown garden snail Cornu aspersum (Müller) (Gastropoda: Pulmonata). Malacologia 2003, 45, 125–132. [Google Scholar]
- Chevalier, L.; Desbuquois, C.; Le Lannic, J.; Charrier, M. Poaceae in the natural diet of the snail Helix aspersa Müller (Gastropoda, Pulmonata). Comptes Rendus de l’Académie des Sci.-Series III-Sci. de la Vie 2001, 324, 979–987. [Google Scholar] [CrossRef]
- Ekin, I.; Şeşen, R. Investigation of the Fatty Acid Contents of Edible Snails Helix lucorum, Eobania vermiculata and Non-Edible Slug Limax flavus. Rec. Nat. Prod. 2017, 11, 562–567. [Google Scholar] [CrossRef]
- Ekin, İ.; Başhan, M.; Şeşen, R. Possible seasonal variation of the fatty acid composition from Melanopsis praemorsa (L., 1758) (Gastropoda: Prosobranchia), from southeast Anatolia, Turkey. Turk. J. Biol. 2011, 35, 203–213. [Google Scholar]
- Harper, P.C. Breeding biology of the fairy prion (Pachyptila turtur) at the Poor Knights Islands, New Zealand. N. Z. J. Zool. 1976, 3, 351–371. [Google Scholar] [CrossRef] [Green Version]
- Givens, D.I.; Gibbs, R.A. Current intakes of EPA and DHA in European populations and the potential of animal-derived foods to increase them: Symposium on ‘How can the n-3 content of the diet be improved?’. Proc. Nutr. Soc. 2008, 67, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of A WHO-FAO Expert Consultation; [Joint WHO-FAO Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases, 2002, Geneva, Switzerland]; WHO technical report series; Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases, Weltgesundheitsorganisation, FAO, Eds.; World Health Organization: Geneva, Switzerland, 2003; ISBN 978-92-4-120916-8. [Google Scholar]
- Weber, J.; Bochi, V.C.; Ribeiro, C.P.; Victório, A.D.M.; Emanuelli, T. Effect of different cooking methods on the oxidation, proximate and fatty acid composition of silver catfish (Rhamdia quelen) fillets. Food Chem. 2008, 106, 140–146. [Google Scholar] [CrossRef]
- Alfaia, C.M.M.; Alves, S.P.; Lopes, A.F.; Fernandes, M.J.E.; Costa, A.S.H.; Fontes, C.M.G.A.; Castro, M.L.F.; Bessa, R.J.B.; Prates, J.A.M. Effect of cooking methods on fatty acids, conjugated isomers of linoleic acid and nutritional quality of beef intramuscular fat. Meat Sci. 2010, 84, 769–777. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. LWT-Food Sci. Technol. 2014, 58, 439–445. [Google Scholar] [CrossRef]
- Rasinska, E.; Rutkowska, J.; Czarniecka-Skubina, E.; Tambor, K. Effects of cooking methods on changes in fatty acids contents, lipid oxidation and volatile compounds of rabbit meat. LWT 2019, 110, 64–70. [Google Scholar] [CrossRef]


| Fatty Acid | T. pisana Raw | T. pisana Boiled | C. aspersum Raw | C. aspersum Boiled | E. vermiculata Raw | E. vermiculata Boiled |
|---|---|---|---|---|---|---|
| Myristic (C14:0) | 0.73 ± 0.01 | 1.06 ± 0.01 | 0.76 ± 0.08 | 0.59 ± 0.03 | 0.81 ± 0.01 | 0.63 ± 0.00 |
| Palmitic (C16:0) | 12.63 ± 0.04 | 15.75 ± 0.16 | 16.02 ± 0.27 | 13.2 4 ± 0.36 | 14.63 ± 0.13 | 13.31 ± 0.00 |
| Margaric (C17:0) | 1.02 ± 0.01 | 1.22 ± 0.07 | 1.13 ± 0.05 | 1.35 ± 0.07 | 1.36 ± 0.07 | 1.04 ± 0.03 |
| Stearic (C18:0) | 5.41 ± 0.08 | 6.31 ± 0.96 | 7.72 ± 0.2 | 7.24 ± 0.14 | 7.66 ± 0.03 | 7.59 ± 0.01 |
| Arachidic (C20:0) | 0.63 ± 0.00 | 0.69 ± 0.04 | 0.71 ± 0.03 | 0.39 ± 0.02 | 0.81 ± 0.02 | 0.37 ± 0.00 |
| Behenic (C22:0) | 0.31 ± 0.00 | 0.29 ± 0.01 | 0.64 ± 0.04 | 0.19 ± 0.00 | 0.31 ± 0.02 | 0.39 ± 0.00 |
| ∑SFA | 20.72 | 25.32 | 26.97 | 23.01 | 25.58 | 23.35 |
| Myristoleic (C14:1) | 0.53 ± 0.00 | 0.59 ± 0.02 | 0.52 ± 0.03 | 0.65 ± 0.03 | 0.23 ± 0.01 | 0.58 ± 0.02 |
| Palmitoleic (C16:1) | 0.50 ± 0.02 | 0.27 ± 0.05 | 0.32 ± 0.08 | 1.35 ± 0.06 | 0.40 ± 0.04 | 0.37 ± 0.01 |
| Eptadecenoic (C17:1) | 0.52 ± 0.01 | 0.54 ± 0.04 | 0.81 ± 0.04 | 1.08 ± 0.04 | 1.01 ± 0.03 | 1.10 ± 0.00 |
| Eicosenoic (C20:1) | 0.37 ± 0.00 | 0.26 ± 0.03 | 0.37 ± 0.04 | 0.50 ± 0.02 | 0.17 ± 0.05 | 0.48 ± 0.00 |
| Erucic (C22:1) | 0.52 ± 0.00 | - | - | - | - | - |
| Oleic (C18:1ω:9) | 28.83 ± 0.08 | 28.83 ± 0.46 | 23.79 ± 1.72 | 29.95 ± 0.46 | 26.03 ± 0.63 | 29.71 ± 0.07 |
| ∑MUFA | 31.27 | 30.49 | 25.81 | 33.53 | 27.86 | 32.24 |
| Linoleic (C18:2 ω6) | 18.78 ± 0.02 | 21.35 ± 0.10 | 22.15 ± 0.13 | 19.07 ± 0.16 | 21.94 ± 0.13 | 19.20 ± 0.02 |
| Linolenic (C18:3 ω3) | 7.64 ± 0.02 | 8.87 ± 0.16 | 15.78 ± 0.09 | 15.14 ± 0.20 | 12.40 ± 0.41 | 15.53 ± 0.00 |
| Eicosadienoic (C20:2) | 5.29 ± 0.02 | 5.44 ± 0.04 | 3.98 ± 0.02 | 4.64 ± 0.02 | 4.21 ± 0.40 | 4.69 ± 0.00 |
| Arachidonic (C20:4) | 6.28 ± 0.08 | 7.17 ± 0.19 | 4.26 ± 0.20 | 4.18 ± 0.01 | 6.40 ± 0.52 | 4.27 ± 0.03 |
| Eicosapentaenoic (C20:5) | 9.85 ± 0.01 | 1.15 ± 0.51 | 0.62 ± 0.07 | 0.40 ± 0.05 | 1.30 ± 0.06 | 0.43 ± 0.01 |
| Docosahexaenoic (C22:6) | 0.18 ± 0.01 | 0.21 ± 0.00 | 0.43 ± 0.01 | 0.13 ± 0.00 | 0.33 ± 0.03 | 0.29 ± 0.01 |
| ∑PUFA | 48.10 | 44.19 | 47.22 | 43.56 | 46.56 | 44.41 |
| ω3/ω6 | 0.58 | 0.30 | 0.55 | 0.56 | 0.43 | 0.58 |
| PUFA/SFA | 2.32 | 1.75 | 1.75 | 1.89 | 1.82 | 1.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluzzo, F.G.; Cammilleri, G.; Ulrici, A.; Calvini, R.; Pulvirenti, A.; Lo Cascio, G.; Macaluso, A.; Vella, A.; Cicero, N.; Amato, A.; et al. Land Snails as a Valuable Source of Fatty Acids: A Multivariate Statistical Approach. Foods 2019, 8, 676. https://0-doi-org.brum.beds.ac.uk/10.3390/foods8120676
Galluzzo FG, Cammilleri G, Ulrici A, Calvini R, Pulvirenti A, Lo Cascio G, Macaluso A, Vella A, Cicero N, Amato A, et al. Land Snails as a Valuable Source of Fatty Acids: A Multivariate Statistical Approach. Foods. 2019; 8(12):676. https://0-doi-org.brum.beds.ac.uk/10.3390/foods8120676
Chicago/Turabian StyleGalluzzo, Francesco Giuseppe, Gaetano Cammilleri, Alessandro Ulrici, Rosalba Calvini, Andrea Pulvirenti, Giovanni Lo Cascio, Andrea Macaluso, Antonio Vella, Nicola Cicero, Antonella Amato, and et al. 2019. "Land Snails as a Valuable Source of Fatty Acids: A Multivariate Statistical Approach" Foods 8, no. 12: 676. https://0-doi-org.brum.beds.ac.uk/10.3390/foods8120676