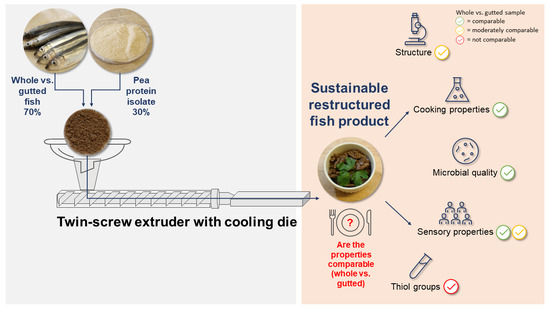
Comparison of Whole and Gutted Baltic Herring as a Raw Material for Restructured Fish Product Produced by High-Moisture Extrusion Cooking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Their Preparation
2.2. Raw Material Composition
2.2.1. Protein Content
2.2.2. Lipid Content
2.2.3. Moisture and Ash Content
2.3. High-Moisture Extrusion Cooking
2.3.1. Preparation of Extrusion Mixtures
2.3.2. Extrusion Parameters
2.4. Determination of Microbial Quality
2.5. Characteristics of the Extrudates
2.5.1. Colour
2.5.2. Microstructure
2.5.3. Mechanical Properties
2.5.4. Cooking Yield and Loss
2.5.5. Free Thiol Groups
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extruder Responses
3.2. Chemical Composition
3.3. Microbiological Quality
3.4. Colour
3.5. Structure Properties
3.6. Sensory Profile
3.7. Cooking Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Wheeler, T.; Von Braun, J. Climate change impacts on global food security. Nat. Syst Chang. Clim. 2013, 341, 479–485. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. Characterization of protein hydrolysates from blue whiting (Micromesistius poutassou) and their application in beverage fortification. Food Chem. 2018, 245, 698–706. [Google Scholar] [CrossRef]
- Cashion, T.; Le Manach, F.; Zeller, D.; Pauly, D. Most fish destined for fishmeal production are food-grade fish. Fish Fish. 2017, 18, 837–844. [Google Scholar] [CrossRef]
- Venugopal, V. Functionality and potential applications of thermostable water dispersions of fish meat. Trends Food Sci. Technol. 1997, 8, 271–276. [Google Scholar] [CrossRef]
- Pihlajamäki, M.; Asikainen, A.; Ignatius, S.; Haapasaari, P.; Tuomisto, J.T. Forage fish as food: Consumer perceptions on baltic herring. Sustainability 2019, 11, 4298. [Google Scholar] [CrossRef] [Green Version]
- Setälä, J.; Saarni, K.; Niukko, J. Fish Market Review 2017. Available online: https://www.luke.fi/wp-content/uploads/2019/05/Fish-market-review-2017.pdf (accessed on 26 February 2020).
- Rantakokko, P.; Peltonen, H.; Leskelä, A.; Hakalax, R.; Myllylä, T.; Lerche, K.-O.; Kiviranta, H. Kalojen Vierasaineiden ja Vesiympäristön Tilan Seurannat Kustannustehokkaammiksi Tutkijoiden ja Kalastuselinkeinon Yhteistyöllä (KALAKAS). Available online: http://www.julkari.fi/bitstream/handle/10024/138790/KALAKAS-Loppuraportti_2019-11-05.pdf?sequence=1&isAllowed=y (accessed on 26 February 2020).
- Tacon, A.G.J.; Metian, M. Fish Matters: Importance of Aquatic Foods in Human Nutrition and Global Food Supply. Rev. Fish. Sci 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Geirsdottir, M.; Sigurgisladottir, S.; Hamaguchi, P.Y.; Thorkelsson, G.; Johannsson, R.; Kristinsson, H.G.; Kristjansson, M.M. Enzymatic Hydrolysis of Blue Whiting (Micromesistius poutassou); Functional and Bioactive Properties. J. Food Sci. 2011, 76, C14–C20. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; Passos, M.G. Restructured fish product from white croacker (Micropogonias furnieri) mince using microbial transglutaminase. Braz. Arch. Biol. Technol. 2010, 53, 987–995. [Google Scholar] [CrossRef]
- Kong, J.; Dougherty, M.P.; Perkins, L.B.; Camire, M.E. Utilization of Smoked Salmon Trim in Extruded Smoked Salmon Jerky. J. Food Sci. 2012, 77, S211–S215. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; Van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Choudhury, G.S.; Gogoi, B.K. Extrusion processing of fish muscle: A review. J. Aquat. Food Prod. Technol. 1996, 4, 37–67. [Google Scholar] [CrossRef]
- Thiébaud, M.; Dumay, E.; Cheftel, J.C. Influence of process variables on the characteristics of a high moisture fish soy protein mix texturized by extrusion cooking. LWT Food Sci. Technol. 1996, 29, 526–535. [Google Scholar] [CrossRef]
- Glaser, C.; Demmelmair, H.; Koletzko, B. High-throughput analysis of total plasma fatty acid composition with direct in situ transesterification. PLoS ONE 2010, 5, e12045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanisamy, M.; Franke, K.; Berger, R.G.; Heinz, V.; Töpfl, S. High moisture extrusion of lupin protein: Influence of extrusion parameters on extruder responses and product properties. J. Sci. Food Agric. 2019, 99, 2175–2185. [Google Scholar] [CrossRef]
- Vogel, B.F.; Venkateswaran, K.; Satomi, M.; Gram, L. Identification of Shewanella baltica as the Most Important H2S-Producing Species during Iced Storage of Danish Marine Fish. Appl. Environ. Microbiol. 2005, 71, 6689–6697. [Google Scholar] [CrossRef] [Green Version]
- Cakli, S.; Kilinc, B.; Cadun, A.; Dincer, T.; Tolasa, S. Effects of gutting and ungutting on microbiological, chemical, and sensory properties of aquacultured sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) stored in ice. Crit. Rev. Food Sci. Nutr. 2006, 46, 519–527. [Google Scholar] [CrossRef]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. 2019, 19, 1021–1030. [Google Scholar] [CrossRef]
- Sathivel, S.; Bechtel, P.J.; Babbitt, J.; Smiley, S.; Crapo, C.; Reppond, K.D.; Prinyawiwatkul, W. Biochemical and functional properties of herring (Clupea harengus) byproduct hydrolysates. J. Food Sci. 2003, 68, 2196–2200. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Muscle tissue: Composition and function. In Food Chemistry, 4th ed.; Springer: Berlin, Germany, 2009; pp. 568–573. [Google Scholar]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Eisner, P.; Schweiggert-Weisz, U. Effect of high moisture extrusion cooking on protein-protein interactions of pea (Pisum sativum L.) protein isolates. Int. J. Food Sci. Technol. 2015, 50, 1390–1396. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Montero, P.; Borderias, J.; Turnay, J.; Leyzarbe, M.A. Characterization of Hake (Merluccius Merluccius, L.) and Trout (Salmo Irideus Gibb) Collagen. J. Agric. Food Chem. 1990, 38, 604–609. [Google Scholar] [CrossRef]
- Benjakul, S.; Morrissey, M.T. Protein Hydrolysates from Pacific Whiting Solid Wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Amino acid, peptides, proteins. In Food Chemistry, 4th ed.; Springer: Berlin, Germany, 2009; pp. 56–57. [Google Scholar]
- Surasani, V.K.R. Application of Food Extrusion Process to Develop Fish Meat-Based Extruded Products. Food Eng. Rev. 2016, 8, 448–456. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Berger, R.G.; Hertel, C. Physico-chemical and nutritional properties of meat analogues based on Spirulina/lupin protein mixtures. Eur. Food Res. Technol. 2019, 245, 1889–1898. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Aganovic, K.; Berger, R.G. Influence of iota carrageenan addition on the properties of soya protein meat analogues. LWT Food Sci. Technol. 2018, 87, 546–552. [Google Scholar] [CrossRef]



| Sample | Protein | Fat | Ash | Moisture |
|---|---|---|---|---|
| R1 | 74.4 ± 0.0 | 5.3 ± 0.1 | 3.7 ± 0.5 | 6.6 ± 0.0 |
| R2 | 14.4 ± 1.6 | 2.3 ± 0.1 | 1.5 ± 0.0 | 81.8 ± 0.1 |
| R3 | 14.2 ± 0.4 | 2.2 ± 0.1 | 2.2 ± 0.1 | 80.3 ± 0.2 |
| ER1 * | 34.9 | 2.5 | 1.6 | 55.7 |
| ER2 * | 32.9 | 3.2 | 2.1 | 54.3 |
| ER3 * | 32.5 | 3.1 | 2.6 | 54.3 |
| Microbial Groups Analyzed | Raw Materials | Extrudates | ||||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | E1 | E2 | E3 | |
| Aerobic heterotrophic bacteria | 2.4 ± 0.7 | 2.1 ± 0.1 | 3.0 ± 0.4 | <1 | 1.4 ± 0.4 | <1 |
| Spore-forming bacteria | <1 | <1 | <1 | <1 | <1 | <1 |
| Bacillus cereus | <1 | <1 | <1 | <1 | <1 | <1 |
| H2S-producing bacteria | <1 | 1.3 ± 0.3 | 1.3 ± 0.6 | <1 | <1 | <1 |
| Enterobacteria | <1 | <1 | <1 | <1 | <1 | <1 |
| Coliformic bacteria | <2 | <2 | <2 | <1 | <1 | <1 |
| Sample | ER1 | ER2 | ER3 | E1 | E2 | E3 |
|---|---|---|---|---|---|---|
| L* (lightness) | 97.7 ± 0.1 a | 75.3 ± 0.5 b | 65.0 ± 0.2 c | 64.7 ± 1.1 a | 62.0 ± 2.2 b | 57.8 ± 1.6 c |
| a* (red/+, green/−) | 0.0 ± 0.0 a | −1.5 ± 0.1 c | −0.6 ± 0.2 b | 4.1 ± 0.2 a | −0.4 ± 0.1 b | 0.4 ± 0.1 c |
| b* (yellow/+, blue/−) | 2.2 ± 0.1 a | −1.1 ± 0.3 b | −2.9 ± 0.2 c | 3.0 ± 1.1 a | −5.9 ± 1.9 b | −6.2 ± 1.4 b |
| Thiols, NaP | 0.6 ± 0.1 c | 6.2 ± 0.2 a | 3.2 ± 0.2 b | 3.2 ± 0.0 b | 5.1 ± 0.6 a | 2.5 ± 0.0 b |
| Thiols, NaP + U | 0.9 ± 0.0 c | 7.0 ± 0.0 a | 3.4 ± 0.0 b | 3.6 ± 0.5 a | 7.8 ± 1.2 a | 5.1 ± 0.3 a |
| Moisture before (%) | - | - | - | 54.2 ± 0.6 b | 56.3 ± 0.2 a | 54.9 ± 1.2 a,b |
| Moisture after (%) | - | - | - | 70.3 ± 0.1 b | 70.2 ± 0.4 b | 77.5 ± 0.9 a |
| Cooking loss (%) | - | - | - | 3.0 ± 0.1 b | 2.9 ± 0.2 b | 5.8 ± 0.3 a |
| Cooking yield (%) | - | - | - | 148.2 ± 1.1 b | 137.8 ± 1.1 c | 183.0 ± 4.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisov, A.; Aisala, H.; Holopainen-Mantila, U.; Alakomi, H.-L.; Nordlund, E.; Honkapää, K. Comparison of Whole and Gutted Baltic Herring as a Raw Material for Restructured Fish Product Produced by High-Moisture Extrusion Cooking. Foods 2020, 9, 1541. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9111541
Nisov A, Aisala H, Holopainen-Mantila U, Alakomi H-L, Nordlund E, Honkapää K. Comparison of Whole and Gutted Baltic Herring as a Raw Material for Restructured Fish Product Produced by High-Moisture Extrusion Cooking. Foods. 2020; 9(11):1541. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9111541
Chicago/Turabian StyleNisov, Anni, Heikki Aisala, Ulla Holopainen-Mantila, Hanna-Leena Alakomi, Emilia Nordlund, and Kaisu Honkapää. 2020. "Comparison of Whole and Gutted Baltic Herring as a Raw Material for Restructured Fish Product Produced by High-Moisture Extrusion Cooking" Foods 9, no. 11: 1541. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9111541