Multiresidue Analysis of Organic UV Filters and UV Stabilizers in Fish of Common Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
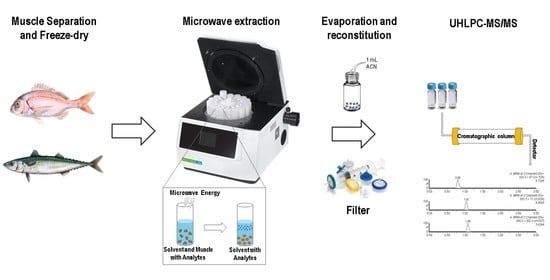
2.2. Sample Collection and Preparation
2.3. Instrumentation
2.4. Chromatographic and Detection Conditions
2.5. Microwave-Assisted Extraction Optimization
2.6. Quality Assurance
3. Results and Discussion
3.1. Validation of the MAE-UHPLC-MS/MS Method
3.2. Presence of UV Filters and BUVSs in Market Fishes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bo, L.; Shengen, Z.; Chang, C. Emerging Pollutants-Part II: Treatment. Water Environ. Res. 2016, 88, 1876–1904. [Google Scholar] [CrossRef] [PubMed]
- Apel, C.; Joerss, H.; Ebinghaus, R. Environmental occurrence and hazard of organic UV stabilizers and UV filters in the sediment of European North and Baltic Seas. Chemosphere 2018, 212, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Langford, K.H.; Reid, M.J.; Fjeld, E.; Øxnevad, S.; Thomas, K.V. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway. Environ. Int. 2015, 80, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Song, Y.K.; Hong, S.H. Benzotriazole-type ultraviolet stabilizers and antioxidants in plastic marine debris and their new products. Sci. Total Environ. 2017, 579, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). HPV Database Search. Available online: https://hpvchemicals.oecd.org/UI/Search.aspx (accessed on 15 June 2020).
- European Chemicals Agency (ECHA). Candidate List of Substances of Very High Concern for Authorisation. Available online: https://echa.europa.eu/candidate-list-table (accessed on 30 July 2020).
- Ziarrusta, H.; Mijangos, L.; Picart-Armada, S.; Irazola, M.; Perera-Lluna, A.; Usobiaga, A.; Prieto, A.; Etxebarria, N.; Olivares, M.; Zuloaga, O. Non-targeted metabolomics reveals alterations in liver and plasma of gilt-head bream exposed to oxybenzone. Chemosphere 2018, 211, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Balázs, A.; Krifaton, C.; Orosz, I.; Szoboszlay, S.; Kovács, R.; Csenki, Z.; Urbányi, B.; Kriszt, B. Hormonal activity, cytotoxicity and developmental toxicity of UV filters. Ecotoxicol. Environ. Saf. 2016, 131, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Quintaneiro, C.; Teixeira, B.; Benedé, J.L.; Chisvert, A.; Soares, A.M.V.M.; Monteiro, M.S. Toxicity effects of the organic UV-filter 4-Methylbenzylidene camphor in zebrafish embryos. Chemosphere 2019, 218, 273–281. [Google Scholar] [CrossRef]
- Li, A.J.; Law, J.C.-F.; Chow, C.-H.; Huang, Y.; Li, K.; Leung, K.S.-Y. Joint Effects of Multiple UV Filters on Zebrafish Embryo Development. Environ. Sci. Technol. 2018, 52, 9460–9467. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Zha, J.; Chen, H.; Martyniuk, C.J.; Liang, X. Transcriptome analysis reveals benzotriazole ultraviolet stabilizers regulate networks related to inflammation in juvenile zebrafish (Danio rerio) brain. Environ. Toxicol. 2019, 34, 112–122. [Google Scholar] [CrossRef]
- Liang, X.; Li, J.; Martyniuk, C.J.; Wang, J.; Mao, Y.; Lu, H.; Zha, J. Benzotriazole ultraviolet stabilizers alter the expression of the thyroid hormone pathway in zebra fish (Danio rerio) embryos. Chemosphere 2017, 182, 22–30. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; He, K.; Gonsior, M.; Hain, E.; Heyes, A.; Clark, C.; Younger, R.; Schmitt-Kopplin, P.; Feerick, A.; Conway, A.; et al. Occurrence and distribution of UV- filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii. Sci. Total Environ. 2019, 670, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.M.P.; Lam, J.C.W.; Ng, T.Y.; Ang, P.O.; Murphy, M.B.; Lam, P.K.S. Occurrence, Distribution, and Fate of Organic UV Filters in Coral Communities. Environ. Sci. Technol. 2017, 51, 4182–4190. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, M.; Li, Z.; Munaron, D.; Le Gall, P.; Casellas, C.; Fenet, H.; Gomez, E. Organic UV filter concentrations in marine mussels from French coastal regions. Sci. Total Environ. 2012, 420, 273–279. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O.; Vallecillos, L.; Cano-Sancho, G.; Domingo, J.L.; Pocurull, E.; Borrull, F.; Maulvault, A.L.; Ferrari, F.; Fernandez-Tejedor, M.; et al. Co-occurrence of musk fragrances and UV-filters in seafood and macroalgae collected in European hotspots. Environ. Res. 2015, 143, 65–71. [Google Scholar] [CrossRef]
- Cunha, S.C.; Trabalón, L.; Jacobs, S.; Castro, M.; Fernandez-Tejedor, M.; Granby, K.; Verbeke, W.; Kwadijk, C.; Ferrari, F.; Robbens, J.; et al. UV-filters and musk fragrances in seafood commercialized in Europe Union: Occurrence, risk and exposure assessment. Environ. Res. 2018, 161, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Shinohara, R.I.; Nakazawa, Y.; Isobe, T.; Sudaryanto, A.; Subramanian, A.; Tanabe, S.; Zakaria, M.P.; Zheng, G.J.; Lam, P.K.S.; et al. Asia-Pacific mussel watch for emerging pollutants: Distribution of synthetic musks and benzotriazole UV stabilizers in Asian and US coastal waters. Mar. Pollut. Bull. 2012, 64, 2211–2218. [Google Scholar] [CrossRef]
- Sang, Z.; Leung, K.S.-Y. Environmental occurrence and ecological risk assessment of organic UV filters in marine organisms from Hong Kong coastal waters. Sci. Total Environ. 2016, 566–567, 489–498. [Google Scholar] [CrossRef]
- Alonso, M.B.; Feo, M.L.; Corcellas, C.; Gago-Ferrero, P.; Bertozzi, C.P.; Marigo, J.; Flach, L.; Carolina, A.; Meirelles, A.C.O.; Carvalho, V.L.; et al. Toxic heritage: Maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil. Environ. Pollut. 2015, 207, 391–402. [Google Scholar] [CrossRef]
- Nakata, H.; Shinohara, R.; Murata, S.; Watanabe, M. Detection of benzotriazole UV stabilizers in the blubber of marine mammals by gas chromatography-high resolution mass spectrometry (GC-HRMS). J. Environ. Monit. 2010, 12, 2088–2092. [Google Scholar] [CrossRef]
- Pacheco-Juárez, J.; Montesdeoca-Esponda, S.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Analysis and occurrence of benzotriazole ultraviolet stabilisers in different species of seaweed. Chemosphere 2019, 236. [Google Scholar] [CrossRef]
- Kim, J.-W.; Ramaswamy, B.R.; Chang, K.-H.; Isobe, T. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3511–3520. [Google Scholar] [CrossRef] [PubMed]
- Emnet, P.; Gaw, S.; Northcott, G.; Storey, B.; Graham, L. Personal care products and steroid hormones in the Antarctic coastal environment associated with two Antarctic research stations, McMurdo Station and Scott Base. Environ. Res. 2015, 136, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Horricks, R.A.; Tabin, S.K.; Edwards, J.J.; Lumsden, J.S.; Marancik, D.P. Organic ultraviolet filters in nearshore waters and in the invasive lionfish (Pterois volitans) in Grenada, West Indies. PLoS ONE 2019, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molins-Delgado, D.; Muñoz, R.; Nogueira, S.; Alonso, M.B.; Torres, J.P.; Malm, O.; Ziolli, R.L.; Hauser-Davis, R.A.; Eljarrat, E.; Barceló, D.; et al. Occurrence of organic UV filters and metabolites in lebranche mullet (Mugil liza) from Brazil. Sci. Total Environ. 2018, 618, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Isobe, T.; Ramaswamy, B.R.; Chang, K.-H.; Amano, A.; Miller, T.M.; Siringan, F.P.; Tanabe, S. Contamination and bioaccumulation of benzotriazole ultraviolet stabilizers in fish from Manila Bay, the Philippines using an ultra-fast liquid chromatography–tandem mass spectrometry. Chemosphere 2011, 85, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.-Y.; Chen, C.-L.; Ding, W.-H. Optimization of matrix solid-phase dispersion for the rapid determination of salicylate and benzophenone-type UV absorbing substances in marketed fish. Food Chem. 2014, 154, 211–216. [Google Scholar] [CrossRef]
- Lu, Z.; De Silva, A.O.; Peart, T.E.; Cook, C.J.; Tetreault, G.R. Tissue Distribution of Substituted Diphenylamine Antioxidants and Benzotriazole Ultraviolet Stabilizers in White Sucker (Catostomus commersonii) from an Urban Creek in Canada. Environ. Sci. Technol. Lett. 2017, 4, 433–438. [Google Scholar] [CrossRef]
- Kim, J.-W.; Chang, K.-H.; Prudente, M.; Viet, P.H.; Takahashi, S.; Tanabe, S.; Kunisue, T.; Isobe, T. Occurrence of benzotriazole ultraviolet stabilizers (BUVSs) in human breast milk from three Asian countries. Sci. Total Environ. 2019, 655, 1081–1088. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; Olmo-Campos, M.; Valeta-Juan, G.; Pleguezuelos-Hernández, V.; Barceló, D.; Díaz-Cruz, M.S. Determination of UV filters in human breast milk using turbulent flow chromatography and babies’ daily intake estimation. Environ. Res. 2018, 161, 532–539. [Google Scholar] [CrossRef]
- Jiménez-Díaz, I.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A. Analytical methods for the determination of personal care products in human samples: An overview. Talanta 2014, 129, 448–458. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Wang, Y.; Wang, T.; Li, X.; Ding, L.; Jiang, G. Evaluation of Soxhlet extraction, accelerated solvent extraction and microwave-assisted extraction for the determination of polychlorinated biphenyls and polybrominated diphenyl ethers in soil and fish samples. Anal. Chim. Acta 2010, 663, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Aufartová, J.; Brabcová, I.; Torres-Padrón, M.E.; Solich, P.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Determination of fluoroquinolones in fishes using microwave-assisted extraction combined with ultra-high performance liquid chromatography and fluorescence detection. J. Food Compos. Anal. 2017, 56, 140–146. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Determination of steroid hormones in fish tissues by microwave-assisted extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry. Food Chem. 2017, 237, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Torres-Padrón, M.E.; Novák, M.; Krchová, L.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Occurrence of benzotriazole UV stabilizers in coastal fishes. J. Environ. Manag. 2020, 269, 110805. [Google Scholar] [CrossRef]
- Santana-Viera, S.; Marzullo, L.; Torres Padrón, M.E.; Del Bubba, M.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Microwave assisted extraction for the determination of antineoplastic compounds in marine fish. J. Food Compos. Anal. 2019, 82, 103241. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Álvarez-Raya, C.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Monitoring and environmental risk assessment of benzotriazole UV stabilizers in the sewage and coastal environment of Gran Canaria (Canary Islands, Spain). J. Environ. Manag. 2019, 233, 567–575. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. UV filters bioaccumulation in fish from Iberian river basins. Sci. Total Environ. 2015, 518, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Jin, J.; Wang, C.; Ou, W.; Tang, C. Multi-target determination of organic ultraviolet absorbents in organism tissues by ultrasonic assisted extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1384, 97–106. [Google Scholar] [CrossRef]
- Ferreira, I.; Gomes-Bispo, A.; Lourenço, H.; Matos, J.; Afonso, C.; Cardoso, C.; Castanheira, I.; Motta, C.; Prates, J.A.M.; Bandarra, N.M. The chemical composition and lipid profile of the chub mackerel (Scomber colias) show a strong seasonal dependence: Contribution to a nutritional evaluation. Biochimie 2020, 178, 181–189. [Google Scholar] [CrossRef]




| Abbreviation | Common Name (IUPAC Name a) | Chemical Structure | CAS Number | Log Kow b |
|---|---|---|---|---|
| 4-MBC | 4-Methylbenzylidene camphor 1,7,7-trimethyl-3-[(4-methylphenyl)methylene]-bicyclo[2.2.1]heptan-2-one |  | 36861-47-9 | 4.95 |
| BP-3 | Benzophenone 3/Oxybenzone (2-hydroxy-4-methoxyphenyl)-phenylmethanone |  | 131-57-7 | 3.79 |
| HMS | Homosalate (3,3,5-trimethylcyclohexyl) 2-hydroxybenzoate |  | 118-56-9 | 6.16 |
| DTS | Drometrizole trisiloxane/Silatrizole 2-(benzotriazol-2-yl)-4-methyl-6-[2-methyl-3-[methyl-bis(trimethylsilyloxy)silyl]propyl]phenol |  | 155633-54-8 | 10.82 |
| OC | Octocrylene 2-ethylhexyl 2-cyano-3,3-diphenylprop-2-enoate |  | 6197-30-4 | 6.88 |
| BM-DBM | Avobenzone/Butyl Methoxy dibenzoylmethane 1-(4-tert-butylphenyl)-3-(4-methoxyphenyl)propane-1,3-dione |  | 70356-09-1 | 4.51 |
| IMC | Amiloxate/ Isoamyl 4-methoxycinnamate 3-methylbutyl (E)-3-(4-methoxyphenyl)prop-2-enoate |  | 71617-10-2 | 4.33 |
| UV-P | 2-(benzotriazol-2-yl)-4-methylphenol |  | 2440-22-4 | 2.99 |
| UV-326 | 2-tert-butyl-6-(5-chlorobenzotriazol-2-yl)-4-methylphenol |  | 3896-11-5 | 5.55 |
| UV-327 | 2,4-ditert-butyl-6-(5-chlorobenzotriazol-2-yl) phenol |  | 3864-99-1 | 6.91 |
| UV-328 | 2-(benzotriazol-2-yl)-4,6-bis(2-methylbutan-2-yl) phenol |  | 25973-55-1 | 7.25 |
| UV-329 | 2-(benzotriazol-2-yl)-4-(2,4,4-trimethylpentan-2-yl) phenol |  | 3147-75-9 | 6.21 |
| UV-360 | 2-(benzotriazol-2-yl)-6-[[3-(benzotriazol-2-yl)-2-hydroxy-5-(2,4,4-trimethylpentan-2-yl) phenyl] methyl]-4-(2,4,4-trimethylpentan-2-yl) phenol |  | 103597-45-1 | 12.5 |
| Total Spain | Catalonia | Canary Islands | |
|---|---|---|---|
| Total fish | 555,343.34 | 84,841.18 | 18,018.60 |
| Hake | 118,151.63 | 19,114.53 | 2484.51 |
| Sardine and anchovy | 58,215.19 | 8827.05 | 644.47 |
| Tuna and bonito | 22,509.33 | 2478.69 | 2498.53 |
| Sole | 30,111.41 | 4477.37 | 961.23 |
| Cod | 41,554.10 | 6667.19 | 1441.08 |
| Mackerel | 13,760.69 | 2052.24 | 1538.56 |
| Compound | Precision (%) | LOD (µg g−1 dw) | LOQ (µg g−1 dw) |
|---|---|---|---|
| 4-MBC | 7.03 | 0.09 | 0.31 |
| BP-3 | 7.42 | 0.03 | 0.09 |
| HMS | 11.8 | 0.06 | 0.18 |
| DTS | 5.01 | 0.03 | 0.09 |
| OC | 6.62 | 0.62 | 2.06 |
| BM-DBM | 5.25 | 0.01 | 0.05 |
| IMC | 4.52 | 0.03 | 0.09 |
| UV-P | 1.88 | 0.34 | 1.14 |
| UV-326 | 8.34 | 0.18 | 0.42 |
| UV-327 | 7.78 | 0.04 | 0.15 |
| UV-328 | 4.62 | 0.03 | 0.09 |
| UV-329 | 3.06 | 0.07 | 0.22 |
| UV-360 | 5.42 | 0.44 | 1.48 |
| Location | Fish Species | Common Name | BP-3 | OC | BM-DBM | UV-328 | UV-329 |
|---|---|---|---|---|---|---|---|
| Canary Islands | Scomber colias | Mackerel | <LOQ | n.d. | <LOQ | n.d. | n.d. |
| Canary Islands | Serranus cabrilla | Comber | <LOQ | n.d. | <LOQ | <LOQ | n.d. |
| Canary Islands | Pagellus erythrinus | Common pandora | n.d. | n.d. | n.d. | n.d. | n.d. |
| Canary Islands | Sarda sarda | Atlantic bonito | n.d. | n.d. | n.d. | n.d. | 0.36 ± 0.02 |
| Catalonia | Gadus morhua | Cod | 0.29 ± 0.02 | n.d. | <LOQ | 0.10 ± 0.01 | <LOQ |
| Catalonia | Solea solea | Sole | 0.09 ± 0.01 | n.d. | <LOQ | 0.30 ± 0.02 | n.d. |
| Catalonia | Merluccius merluccius | Hake | <LOQ | n.d. | <LOQ | n.d. | n.d. |
| Catalonia | Sardina pilchardus | Sardine | 0.07 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| Catalonia | Thunnus thynnus | Tuna | 0.68 ± 0.05 | <LOQ | n.d. | n.d. | 0.52 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimeno-Monforte, S.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Castro, Ó.; Pocurull, E.; Borrull, F. Multiresidue Analysis of Organic UV Filters and UV Stabilizers in Fish of Common Consumption. Foods 2020, 9, 1827. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9121827
Gimeno-Monforte S, Montesdeoca-Esponda S, Sosa-Ferrera Z, Santana-Rodríguez JJ, Castro Ó, Pocurull E, Borrull F. Multiresidue Analysis of Organic UV Filters and UV Stabilizers in Fish of Common Consumption. Foods. 2020; 9(12):1827. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9121827
Chicago/Turabian StyleGimeno-Monforte, Sandra, Sarah Montesdeoca-Esponda, Zoraida Sosa-Ferrera, José Juan Santana-Rodríguez, Óscar Castro, Eva Pocurull, and Francesc Borrull. 2020. "Multiresidue Analysis of Organic UV Filters and UV Stabilizers in Fish of Common Consumption" Foods 9, no. 12: 1827. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9121827