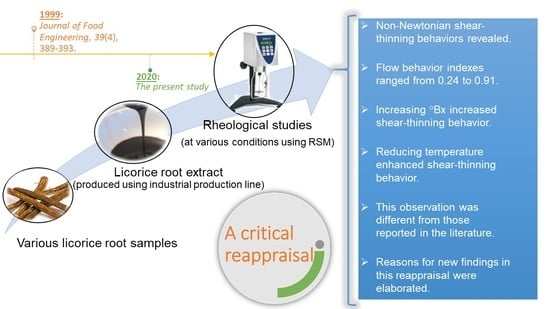
Rheological Behavior of Glycyrrhiza glabra (Licorice) Extract as a Function of Concentration and Temperature: A Critical Reappraisal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chemical Analysis of Samples
2.3. Rheological Studies
2.4. The Temperature Dependency of Extracts Rheological Behavior
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Licorice Extracts
3.2. Rheological Properties of Extracts
3.2.1. Flow Behaviors
3.2.2. Effects of Temperature and Degrees Brix on Licorice Viscosity
3.2.3. Flow Behavior Index of Extracts
3.3. Response Surface Methodology Results
3.4. Activation Energy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Geng, Y.; Song, M.; Zhang, P.; Hou, J.; Wang, W. Genetic structure and diversity of Glycyrrhiza populations based on transcriptome SSR markers. Plant. Mol. Biol. Report. 2019, 37, 401–412. [Google Scholar] [CrossRef]
- Mutaillifu, P.; Bobakulov, K.; Abuduwaili, A.; Huojiaaihemaiti, H.; Nuerxiati, R.; Aisa, H.A.; Yili, A. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macromol. 2020, 144, 751–759. [Google Scholar] [CrossRef]
- Rohinishree, Y.S.; Negi, P.S. Effect of licorice extract on cell viability, biofilm formation and exotoxin production by Staphylococcus aureus. J. Food Sci. Technol. 2016, 53, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Salawu, S.O.; Ibukun, E.O.; Esan, I.A. Nutraceutical values of hot water infusions of moringa leaf (Moringa oleifera) and licorice root (Glycyrrhiza glabra) and their effects on liver biomarkers in Wistar rats. J. Food Meas. Charact. 2019, 13, 602–613. [Google Scholar] [CrossRef]
- Karkanis, A.; Martins, N.; Petropoulos, S.A.; Ferreira, I.C.F.R. Phytochemical composition, health effects, and crop management of liquorice (Glycyrrhiza glabra L.): A medicinal plant. Food Rev. Int. 2018, 34, 182–203. [Google Scholar] [CrossRef] [Green Version]
- Taarji, N.; Bouhoute, M.; Fainassi, F.; Hafidi, A.; Kobayashi, I.; Neves, M.A.; Tominaga, K.; Isoda, H.; Nakajima, M. Interfacial and emulsifying properties of purified glycyrrhizin and non-purified glycyrrhizin-rich extracts from liquorice root (Glycyrrhiza glabra). Food Chem. 2021, 337. [Google Scholar] [CrossRef]
- Sinha, S.K.; Prasad, S.K.; Islam, M.A.; Gurav, S.S.; Patil, R.B.; AlFaris, N.A.; Aldayel, T.S.; AlKehayez, N.M.; Wabaidur, S.M.; Shakya, A. Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and non-structural protein-15: A pharmacoinformatics study. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Šibul, F.; Sinka, I.; Zupko, I.; Ocsovszki, I.; Jovanović-Šanta, S. Chemical composition, antioxidant and anticancer activity of licorice from Fruska Gora locality. Ind. Crops Prod. 2018, 112, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Gavahian, M.; Chu, Y.-H.H.; Sastry, S. Extraction from food and natural products by moderate electric field: Mechanisms, benefits, and potential industrial applications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1040–1052. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Zhao, S.; Yang, S.; Lin, X.; He, X.; Wei, X.; Song, Q.; Li, R.; Fu, C.; Zhang, J.; et al. An “essential herbal medicine”—Licorice: A review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol. 2020, 249, 112439. [Google Scholar] [CrossRef]
- Karaaslan, İ.; Dalgıç, A.C. Spray drying of liquorice (Glycyrrhiza glabra) extract. J. Food Sci. Technol. 2014, 51, 3014–3025. [Google Scholar] [CrossRef] [Green Version]
- Gabriele, D.; Curcio, S.; DeCindio, B. Optimal design of single-screw extruder for liquorice candy production: A rheology based approach. J. Food Eng. 2001, 48, 33–44. [Google Scholar] [CrossRef]
- ISIRIS (Institute of Standards and Industrial Research of Iran), Tehran, Report No. 2343. Available online: www.isiri.gov.ir (accessed on 24 September 2018).
- Junqueira, L.A.; da Silva, V.M.; Amaral, T.N.; Prado, M.E.T.; de Resende, J.V. Effects of temperature and concentration on the rheological properties of mucilage extracted from Pereskia aculeata Miller. J. Food Meas. Charact. 2019, 13, 2549–2562. [Google Scholar] [CrossRef]
- Kamboj, R.; Nayik, G.A.; Bera, M.B.; Nanda, V. Sugar profile and rheological behaviour of four different Indian honey varieties. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Shu, G.; Chen, H. Production of Angiotensin-I-Converting Enzyme inhibitory peptide from goat milk casein: Optimization conditions of complex protease hydrolysate by response surface methodology and purification. Emirates J. Food Agric. 2018, 742. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, L.; Liu, J.; Zhang, S.; Sun, X.; Al-Marashdeh, O. Effects of licorice extract supplementation on feed intake, digestion, rumen function, blood indices and live weight gain of karakul sheep. Animals 2019, 9, 279. [Google Scholar] [CrossRef] [Green Version]
- Rossow, M.; Ludewig, M.; Braun, P.G. Effect of cold atmospheric pressure plasma treatment on inactivation of Campylobacter jejuni on chicken skin and breast fillet. LWT—Food Sci. Technol. 2018, 91, 265–270. [Google Scholar] [CrossRef]
- Maskan, M. Rheological behaviour of liquorice (Glycyrrhiza glabra). J. Food Eng. 1999, 39, 389–393. [Google Scholar] [CrossRef]
- Farahnaky, A.; Azizi, R.; Gavahian, M. Accelerated texture softening of some root vegetables by Ohmic heating. J. Food Eng. 2012, 113, 275–280. [Google Scholar] [CrossRef]
- Zhou, P.; Eid, M.; Xiong, W.; Ren, C.; Ai, T.; Deng, Z.; Li, J.; Li, B. Comparative study between cold and hot water extracted polysaccharides from Plantago ovata seed husk by using rheological methods. Food Hydrocoll. 2020, 101. [Google Scholar] [CrossRef]
- Gavahian, M.; Munekata, P.E.S.; Eş, I.; Lorenzo, J.M.; Mousavi Khaneghah, A.; Barba, F.J. Emerging techniques in bioethanol production: From distillation to waste valorization. Green Chem. 2019, 21, 1171–1185. [Google Scholar] [CrossRef]
- Gavahian, M.; Farahnaky, A. Ohmic-assisted hydrodistillation technology: A review. Trends Food Sci. Technol. 2018, 72, 153–161. [Google Scholar] [CrossRef]
- Deshmukh, P.S.; Manjunatha, S.S.; Raju, P.S. Rheological behaviour of enzyme clarified sapota (Achras sapota L.) juice at different concentration and temperatures. J. Food Sci. Technol. 2015, 52, 1896–1910. [Google Scholar] [CrossRef] [Green Version]



| Licorice Sample Code | Moisture (%) | Ash (%) | Gums and Starch (%) | Glycyrrhizin (%) | Insoluble in Cold Water (%) | Insoluble in Hot Water (%) |
|---|---|---|---|---|---|---|
| 1 | 53.28 ± 0.35 a | 8.38 ± 0.27 cd | 23.79 ± 0.73 c | 16.39 ± 0.23 bc | 20.82 ± 0.84 g | 0.87 ± 0.22 ab |
| 2 | 62.74 ± 0.13 gh | 9.63 ± 0.06 e | 21.93 ± 0.73 a | 22.77 ± 1.01 d | 3.34 ± 0.52 a | 1.38 ± 0.59 abc |
| 3 | 61.77 ± 0.08 de | 8.15 ± 0.36 abcd | 31.37 ± 0.13 fg | 16.35 ± 0.32 bc | 13.52 ± 0.75 de | 1.54 ± 0.84 bcd |
| 4 | 61.83 ± 0.13 def | 8.55 ± 0.09 d | 25.46 ± 0.65 d | 17.31 ± 0.30 c | 50.11 ± 0.49 c | 2.69 ± 0.52 d |
| 5 | 62.40 ± 0.54 fgh | 7.47 ± 0.47 a | 29.15 ± 0.71 e | 17.77 ± 0.35 c | 12.38 ± 0.17 cd | 2.21 ± 0.38 bcd |
| 6 | 62.95 ± 10.0 h | 8.56 ± 0.29 d | 23.43 ± 0.83 bc | 22.63 ± 1.69 d | 8.38 ± 0.50 b | 0.48 ± 0.16 ab |
| 7 | 61.51 ± 0.33 d | 8.30 ± 0.21 bcd | 23.66 ± 0.37 c | 15.91 ± 1.48 abc | 10.61 ± 0.39 c | 1.45 ± 0.73 abc |
| 8 | 62.17 ± 0.03 efg | 7.96 ± 0.35 abcd | 22.05 ± 0.72 ab | 14.99 ± 0.18 ab | 18.56 ± 0.24 f | 0.66 ± 0.25 ab |
| 9 | 60.34 ± 0.36 c | 7.64 ± 0.37 ab | 25.65 ± 0.04 d | 14.06 ± 1.15 a | 23.74 ± 1.09 h | 0.82 ± 0.16 ab |
| 10 | 60.63 ± 0.16 c | 7.74 ± 0.13 abc | 32.45 ± 0.23 g | 16.23 ± 0.27 bc | 18.28 ± 1.52 f | 0.30 ± 0.13 ab |
| 11 | 61.52 ± 0.12 d | 7.44 ± 0.34 a | 30.54 ± 0.23 ef | 16.63 ± 0.47 bc | 50.18 ± 0.41 f | 0.30 ± 0.13 ab |
| 12 | 58.56 ± 0.14 b | 8.09 ± 0.12 abcd | 29.62 ± 0.14 e | 17.51 ± 0.26 c | 14.98 ± 0.34 e | 0.17 ± 0.05 a |
| Licorice Sample Code | °Brix | ||||
|---|---|---|---|---|---|
| 15 | 20 | 30 | 40 | 45 | |
| 30 °C | |||||
| 1 | 11.39 ± 1.50 a | 35.75 ± 3.35 a | 670.45 ± 121.05 b | 1010.80 ± 97.25 b | 3985.00 ± 36.00 d |
| 2 | 27.62 ± 4.58 bc | 29.52 ± 7.11 a | 46.95 ± 2.75 a | 85.25 ± 0.25 a | 139.85 ± 0.85 a |
| 3 | 42.90 ± 2.30 d | 206.05 ± 2.95 e | 2887.50 ± 173.50 d | 5411.50 ± 404.50 i | ND |
| 4 | 31.10 ± 0.10 cd | 280.65 ± 11.15 f | 499.35 ± 33.05 b | 2947.50 ± 101.50 f | 7781.00 ± 21.00 h |
| 5 | 83.80 ± 16.80 e | 160.95 ± 4.05 c | 610.95 ± 34.95 b | 4751.50 ± 63.50 h | 7504.50 ± 47.50 g |
| 6 | 15.00 ± 3.30 ab | 37.85 ± 2.15 a | 652.90 ± 19.40 b | 4740.00 ± 63.50 h | 7440.50 ± 105.50 g |
| 7 | 84.90 ± 0.60 e | 101.25 ± 0.45 b | 333.50 ± 31.10 ab | 1866.50 ± 9.50 d | 3086.80 ± 61.77 c |
| 8 | 18.40 ± 0.70 abc | 40.15 ± 0.45 a | 264.90 ± 27.70 ab | 3728.50 ± 29.50 g | 5220 ± 46.35 e |
| 9 | 19.55 ± 0.45 abc | 208.00 ± 6.85 e | 441.00 ± 31.40 ab | 1522.50 ± 90.50 c | 2847.50 ± 125.50 b |
| 10 | 143.70 ± 1.30 g | 421.65 ± 6.85 g | 1454.50 ± 105.50 c | 8019.00 ± 40.00 j | ND |
| 11 | 87.10 ± 1.80 e | 188.85 ± 0.75 d | |||
| 12 | 115.70 ± 0.60 f | 197.50 ± 13.50 de | |||
| 60 °C | |||||
| 1 | 5.80 ± 0.840 a | 15.06 ± 2.56 a | 230.90 ± 3.70 e | 491.55 ± 11.15 c | 4637.50 ± 159.50 f |
| 2 | 4.82 ± 4.20 a | 10.17 ± 1.73 a | 29.30 ± 3.60 a | 36.30 ± 4.80 a | 50.30 ± 6.00 ab |
| 3 | 10.86 ± 4.94 a | 59.90 ± 6.70 c | 315.95 ± 3.15 f | 5863.50 ± 8.50 h | ND |
| 4 | 22.95 ± 1.45 a | 75.55 ± 2.95 d | 175.20 ± 14.30 d | 206.96 ± 0.64 b | 1847.50 ± 122.50 d |
| 5 | 54.70 ± 7.20 b | 124.60 ± 12.80 e | 212.00 ± 21.50 e | 3779.50 ± 120.50 f | 7343.00 ± 689.00 g |
| 6 | 4.67 ± 0.47 a | 15.10 ± 2.10 a | 69.95 ± 1.15 b | 969.85 ± 4.25 d | 3124.00 ± 223.00 e |
| 7 | 13.70 ± 1.50 a | 60.80 ± 5.20 c | 70.80 ± 0.20 b | 408.15 ± 7.05 c | 544.70 ± 8.40 b |
| 8 | 25.25 ± 1.35 a | 57.50 ± 0.70 a | 103.45 ± 0.85 c | 476.20 ± 25.10 c | 4738.00 ± 12.20 f |
| 9 | 14.96 ± 8.54 a | 39.35 ± 0.15 b | 408.50 ± 20.00 g | 1005.50 ± 17.50 d | 1132.00 ± 77.14 c |
| 10 | 49.00 ± 25.30 b | 78.10 ± 0.60 d | 959.30 ± 0.50 i | 4381.00 ± 4.00 g | ND |
| 11 | 20.45 ± 3.04 a | 53.05 ± 2.65 c | 824.05 ± 22.55 h | 5839.00 ± 138.00 h | ND |
| 12 | 18.20 ± 1.10 a | 20.15 ± 2.35 a | 941.90 ± 7.50 i | 2389.00 ± 98.00 e | 3437.50 ± 80.00 e |
| Licorice Sample Code | °Brix | ||||
|---|---|---|---|---|---|
| 15 | 20 | 30 | 40 | 45 | |
| 30 °C | |||||
| 1 | 0.47 ± 0.05 a | 0.71 ± 0.02 hi | 0.49 ± 0.04 d | 0.63 ± 0.02 f | 0.51 ± 0.01 d |
| 2 | 0.78 ± 0.02 c | 0.77 ± 0.03 h | 0.78 ± 0.02 g | 0.79 ± 0.01 g | 0.80 ± 0.00 f |
| 3 | 0.56 ± 0.01 ab | 0.47 ± 0.00 b | 0.26 ± 0.01a | 0.37 ± 0.01 bc | ND |
| 4 | 0.55 ± 0.01 ab | 0.34 ± 0.01 a | 0.39 ± 0.01 c | 0.38 ± 0.01 bc | 0.24 ± 0.00 a |
| 5 | 0.47 ± 0.01 a | 0.57 ± 0.01 f | 0.49 ± 0.01 d | 0.39 ± 0.02 c | 0.42 ± 0.02 c |
| 6 | 0.76 ± 0.01 c | 0.72 ± 0.01 i | 0.40 ± 0.01 c | 0.30 ± 0.01 a | 0.33 ± 0.00 b |
| 7 | 0.51 ± 0.01 a | 0.48 ± 0.01 bc | 0.45 ± 0.01 d | 0.42 ± 0.01 d | 0.43 ± 0.01 c |
| 8 | 0.72 ± 0.01 bc | 0.69 ± 0.01 g | 0.55 ± 0.01 e | 0.36 ± 0.01 b | 0.41 ± 0.02 c |
| 9 | 0.78 ± 0.01 c | 0.52 ± 0.00 d | 0.64 ± 0.01 f | 0.63 ± 0.01 f | 0.58 ± 0.01 e |
| 10 | 0.52 ± 0.00 a | 0.50 ± 0.01 cd | 0.47 ± 0.01 d | 0.32 ± 0.01 a | ND |
| 11 | 0.55 ± 0.00 b | 0.55 ± 0.01 ef | 0.32 ± 0.01 b | 0.38 ± 0.00 bc | ND |
| 12 | 0.49 ± 0.00 a | 0.53 ± 0.00 de | 0.52 ± 0.01 e | 0.50 ± 0.00 e | 0.43 ± 0.00 c |
| 60 °C | |||||
| 1 | 0.71 ± 0.06 b | 0.82 ± 0.03 f | 0.62 ± 0.01 d | 0.625 ± 0.01 g | 0.40 ± 0.01 c |
| 2 | 0.68 ± 0.05 b | 0.64 ± 0.05 cd | 0.46 ± 0.04 a | 0.82 ± 0.04 h | 0.89 ± 0.02 h |
| 3 | 0.67 ± 0.01 b | 0.63 ± 0.02 c | 0.63 ± 0.01 d | 0.28 ± 0.01 a | ND |
| 4 | 0.60 ± 0.01 b | 0.47 ± 0.02 b | 0.51 ± 0.03 b | 0.80 ± 0.01 h | 0.48 ± 0.01 f |
| 5 | 0.32 ± 0.02 a | 0.39 ± 0.02 a | 0.63 ± 0.03 d | 0.32 ± 0.01 b | 0.27 ± 0.01 a |
| 6 | 0.91 ± 0.01 c | 0.80 ± 0.01 ef | 0.74 ± 0.01 e | 0.49 ± 0.00 e | 0.39 ± 0.01 c |
| 7 | 0.65 ± 0.00 b | 0.42 ± 0.00 a | 0.67 ± 0.00 d | 0.58 ± 0.00 f | 0.62 ± 0.01 g |
| 8 | 0.66 ± 0.01 b | 0.77 ± 0.01 ef | 0.63 ± 0.01 d | 0.59 ± 0.00 f | 0.37 ± 0.01 b |
| 9 | 0.63 ± 0.14 b | 0.75 ± 0.00 e | 0.56 ± 0.00 bc | 0.47 ± 0.02 de | 0.42 ± 0.01 d |
| 10 | 0.60 ± 0.12 b | 0.65 ± 0.00 cd | 0.46 ± 0.00 a | 0.39 ± 0.00 c | ND |
| 11 | 0.38 ± 0.00 a | 0.69 ± 0.01 d | 0.46 ± 0.00 a | 0.27 ± 0.00 a | ND |
| 12 | 0.67 ± 0.01 b | 0.77 ± 0.02 ef | 0.50 ± 0.00 ab | 0.44 ± 0.01 d | 0.45 ± 0.00 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasiri, L.; Gavahian, M.; Majzoobi, M.; Farahnaky, A. Rheological Behavior of Glycyrrhiza glabra (Licorice) Extract as a Function of Concentration and Temperature: A Critical Reappraisal. Foods 2020, 9, 1872. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9121872
Nasiri L, Gavahian M, Majzoobi M, Farahnaky A. Rheological Behavior of Glycyrrhiza glabra (Licorice) Extract as a Function of Concentration and Temperature: A Critical Reappraisal. Foods. 2020; 9(12):1872. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9121872
Chicago/Turabian StyleNasiri, Laleh, Mohsen Gavahian, Mahsa Majzoobi, and Asgar Farahnaky. 2020. "Rheological Behavior of Glycyrrhiza glabra (Licorice) Extract as a Function of Concentration and Temperature: A Critical Reappraisal" Foods 9, no. 12: 1872. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9121872