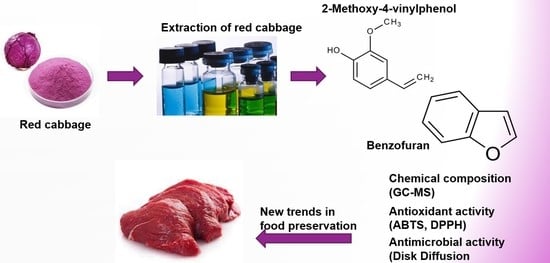
Bioactive Potential of 2-Methoxy-4-vinylphenol and Benzofuran from Brassica oleracea L. var. capitate f, rubra (Red Cabbage) on Oxidative and Microbiological Stability of Beef Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction Procedure
2.2. Total Phenol (TP), Total Flavonoid (TF) Content, and Antioxidant Activity of Red Cabbage
2.2.1. TP and TF Content
2.2.2. Antioxidant Activity Assays
2.3. GC-MS Analysis
2.4. Antimicrobial Activity
2.4.1. Microorganisms
2.4.2. Antimicrobial Assay
2.4.3. Determination of Minimum Inhibitory Concentration (MIC)
2.4.4. Thermal Stability
2.5. Docking Method
2.6. Cytotoxicity Assay
2.7. In Vivo Analysis of Cytotoxicity
2.7.1. C. elegans Culture Conditions
2.7.2. Chemotaxis Assay
2.7.3. Colonization Assay (In Vivo Antimicrobial Activity)
2.8. Application of RCC Extract on Raw Beef Meat
2.8.1. Beef Sample Preparation and Storage Conditions
2.8.2. Microbial Analysis
2.8.3. Physiochemical Analysis
pH Analysis
Color Measurements
Texture Profile Analysis (TPA)
Moisture Analysis
Thiobarbituric Acid Reactive Substances (TBARS)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Antimicrobial Activity
3.2. Phenolic and Flavonoid Content
3.3. Antioxidant Activity
3.4. GC-MS Analysis
3.5. In Silico Molecular Docking Mechanism
3.6. Effect of RCC on C. Elegans (In Vivo) Model against E. coli O157:H7
3.7. Shelf Life Study
3.7.1. Changes in Microbial Profile
3.7.2. Changes in pH
3.7.3. Changes in Moisture
3.7.4. Changes in TBARS
3.7.5. Color Analysis
3.7.6. Textural Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omoruyi, I.M.; Uwadiae, E.; Mulade, G.; Omoruku, E. Shiga toxin producing strains of Escherichia coli (STEC) associated with beef products and its potential pathogenic effect. Microbiol. Res. J. Int. 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agri. 2019, 99, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Hintz, T.; Matthews, K.K.; Di, R. The use of plant antimicrobial compounds for food preservation. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [Green Version]
- Rubab, M.; Chellia, R.; Saravanakumar, K.; Mandava, S.; Khan, I.; Tango, C.N.; Hussain, M.S.; Daliri, E.B.-M.; Kim, S.-H.; Ramakrishnan, S.R.; et al. Preservative effect of Chinese cabbage (Brassica rapa subsp. pekinensis) extract on their molecular docking, antioxidant and antimicrobial properties. PLoS ONE 2018, 13, e0203306. [Google Scholar] [CrossRef] [Green Version]
- Bakari, S.; Ncir, M.; Felhi, S.; Hajlaoui, H.; Saoudi, M.; Gharsallah, N.; Kadri, A. Chemical composition and in vitro evaluation of total phenolic, flavonoid, and antioxidant properties of essential oil and solvent extract from the aerial parts of Teucrium polium grown in Tunisia. Food Sci. Biotechnol. 2015, 24, 1943–1949. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G.; et al. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Franco-Ulloa, S.; La Sala, G.; Miscione, G.; De Vivo, M. Novel bacterial topoisomerase inhibitors exploit Asp83 and the intrinsic flexibility of the DNA gyrase binding site. Int. J. Mol. Sci. 2018, 19, 453. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, E.; Akhtar, S.; Khan, M.K.A.; Lohani, M.; Arif, J.M.; Siddiqui, M.H. Molecular docking analysis of aplysin analogs targeting survivin protein. Bioinformation 2017, 13, 293. [Google Scholar] [CrossRef] [Green Version]
- Chelliah, R.; Choi, J.-G.; Hwang, S.-b.; Park, B.-J.; Daliri, E.B.-M.; Kim, S.-H.; Wei, S.; Ramakrishnan, S.R.; Oh, D.-H. In vitro and in vivo defensive effect of probiotic LAB against Pseudomonas aeruginosa using Caenorhabditis elegans model. Virulence 2018, 9, 1489–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Tango, C.N.; Oh, D.-H. Development and evaluation of chitosan and its derivative for the shelf life extension of beef meat under refrigeration storage. Int. J. Food Sci. Technol. 2017, 52, 1111–1121. [Google Scholar] [CrossRef]
- Barekat, S.; Soltanizadeh, N. Improvement of meat tenderness by simultaneous application of high-intensity ultrasonic radiation and papain treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 223–229. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2012. [Google Scholar]
- Du, M.; Ahn, D. Effect of antioxidants on the quality of irradiated sausages prepared with turkey thigh meat. Poult. Sci. 2002, 81, 1251–1256. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, K.-T.; Nah, S.-Y.; Chung, M.-S.; Cho, S.; Paik, H.-D. Antimicrobial and antioxidative effects of onion peel extracted by the subcritical water. Food Sci. Biotechnol. 2011, 20, 543–548. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Kim, S.-J.; Cho, A.R.; Han, J. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control 2013, 29, 112–120. [Google Scholar] [CrossRef]
- Köksal, E.; Bursal, E.; Gülçin, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.C.; Alwasel, S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. B 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Nićiforović, N.; Mihailović, V.; Mašković, P.; Solujić, S.; Stojković, A.; Muratspahić, D.P. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem. Toxicol. 2010, 48, 3125–3130. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Lee, H.-S.; Kang, I.-J.; Won, M.-H.; You, S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Martysiak-Żurowska, D.; Wenta, W. A comparison of ABTS and DPPH methods for assessing the total antioxidant capacity of human milk. Acta Scient. Polon. Technol. Aliment. 2012, 11, 83–89. [Google Scholar]
- Udayaprakash, N.; Ranjithkumar, M.; Deepa, S.; Sripriya, N.; Al-Arfaj, A.A.; Bhuvaneswari, S. Antioxidant, free radical scavenging and GC–MS composition of Cinnamomum iners Reinw. ex Blume. Ind. Crops Prod. 2015, 69, 175–179. [Google Scholar] [CrossRef]
- Chemonges, S. The recognition of LpxC inhibitors as potential antibiotics could revolutionise the management of sepsis in veterinary patients if their unknown biological properties are widely evaluated in suitable animal models. Int. J. Vet. Sci. Med. 2014, 2, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Collin, F.; Karkare, S.; Maxwell, A. Exploiting bacterial DNA gyrase as a drug target: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 92, 479–497. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, T.J.; Nayar, A.; Newman, J.V.; Hopkins, S.; Stone, G.G.; Johnstone, M.; Shapiro, A.B.; Cronin, M.; Reck, F.; Ehmann, D.E. NBTI 5463 is a novel bacterial type II topoisomerase inhibitor with activity against Gram-negative bacteria and in vivo efficacy. Antimicrob. Agents Chemother. 2014, 58, 2657–2664. [Google Scholar] [CrossRef] [Green Version]
- Hameed, I.H.; Hussein, H.J.; Kareem, M.A.; Hamad, N.S. Identification of five newly described bioactive chemical compounds in methanolic extract of Mentha viridis by using gas chromatography-mass spectrometry (GC-MS). J. Pharmacog. Phytother. 2015, 7, 107–125. [Google Scholar]
- Ibibia, E.T.; Olabisi, K.N.; Oluwagbemiga, O.S. Gas chromatography-mass spectrometric analysis of methanolic leaf extracts of lannea kerstingii and nauclea diderrichii, two medicinal plants used for the treatment of gastrointestinal tract infections. Asian J. Pharm. Clin. Res. 2016, 9, 179–182. [Google Scholar]
- Saleem, H.; Htar, T.T.; Naidu, R.; Nawawi, N.S.; Ahmad, I.; Ashraf, M.; Ahemad, N. Biological, chemical and toxicological perspectives on aerial and roots of Filago germanica (L.) huds: Functional approaches for novel phyto-pharmaceuticals. Food Chem. Toxicol. 2019, 123, 363–373. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B.; Aliakbarlu, J.; Tajik, H. Effect of pomegranate juice dipping and chitosan coating enriched with Zataria multiflora Boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 280–287. [Google Scholar] [CrossRef]
- da Cruz Cabral, L.; Fernández Pinto, V.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Malikarjunan, K.; Roohinejad, S. Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. Meat Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Koné, A.P.; Desjardins, Y.; Gosselin, A.; Cinq-Mars, D.; Guay, F.; Saucier, L. Plant extracts and essential oil product as feed additives to control rabbit meat microbial quality. Meat Sci. 2019, 150, 111–121. [Google Scholar] [CrossRef]
- Fernández-López, J.; Zhi, N.; Aleson-Carbonell, L.; Pérez-Alvarez, J.A.; Kuri, V. Antioxidant and antibacterial activities of natural extracts: Application in beef meatballs. Meat Sci. 2005, 69, 371–380. [Google Scholar] [CrossRef]
- Nieto, G.; Díaz, P.; Bañón, S.; Garrido, M.D. Dietary administration of ewe diets with a distillate from rosemary leaves (Rosmarinus officinalis L.): Influence on lamb meat quality. Meat Sci. 2010, 84, 23–29. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, H.J.; Cheong, S.H.; Kim, Y.-S.; Kim, S.-E.; Hwang, J.-W.; Lee, J.-S.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Antioxidative effect of recombinant ice-binding protein (rLeIBP) from Arctic yeast Glaciozyma sp. on lipid peroxidation of Korean beef. Proc. Biochem. 2015, 50, 2099–2104. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Guo, X. Effects of antimicrbial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci. Human Illnes 2016, 5, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-W.; Cheong, S.H.; Kim, Y.-S.; Lee, J.-W.; You, B.-I.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Effects of dietary supplementation of oriental herbal medicine residue and methyl sulfonyl methane on the growth performance and meat quality of ducks. Anim. Prod. Sci. 2017, 57, 948–957. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Rahim, H.A.; Ghazali, R. The application of near-infrared spectroscopy for poultry meat grading. In Proceedings of the 2012 IEEE 8th International Colloquium on Signal Processing and its Applications, Melaka, Malaysia, 23–25 March 2012; pp. 58–62. [Google Scholar]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estevez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]





| List of Microorganisms | Zone of Inhibition Diameter (mm) | ||||||
|---|---|---|---|---|---|---|---|
| CE | TE | DE | EEE | EtE | ME | DWE | |
| Gram-negative bacteria | |||||||
| Salmonella. enterica typhimurium ATCC 14,028 | 12.00 ± 0.01b | 12.00 ± 0.04b | 10.00 ± 0.02a | - | 11.00 ± 0.03ab | 10.00 ± 0.05a | - |
| Escherichia. coli ATCC 35,150 | 13.00 ± 0.02c | 11.00 ± 0.03b | 10.00 ± 0.05ab | - | 10.00 ± 0.05ab | 09.00 ± 0.06a | - |
| Escherichia. coli ATCC 43,894 | 11.00 ± 0.03b | 10.00 ± 0.02ab | 10.00 ± 0.04ab | - | 11.00 ± 0.03b | 09.00 ± 0.06a | - |
| Gram-positive bacteria | |||||||
| Staphylococcus. aureus ATCC 13,150 | 14.00 ± 0.04bc | 11.00 ± 0.02ab | 13.00 ± 0.04b | - | - | 10.00 ± 0.05a | - |
| Staphylococcus. aureus ATCC 12,600 | 11.00 ± 0.02ab | 11.00 ± 0.05ab | 10.00 ± 0.06a | - | 11.00 ± 0.07ab | - | - |
| Listeria. monocytogenes ATCC 19,118 | 12.00 ± 0.02b | 12.00 ± 0.05b | 10.00 ± 0.05a | - | 13.00 ± 0.02c | - | - |
| Bacillus. cereus ATCC 14,579 | 14.00 ± 0.03b | 12.00 ± 0.05ab | 11.00 ± 0.06a | - | 11.00 ± 0.02a | - | - |
| Fungi | |||||||
| Candida. albicans KCTC 7965 | 15.00 ± 0.01b | - | 13.00 ± 0.05a | 14.00 ± 0.03ab | - | - | - |
| Aspergillus. fumigatus KCTC 6145 | 08.50 ± 0.02a | - | - | 10.00 ± 0.05b | - | - | - |
| Aspergillus. flavus var. flavus KCTC 6143 | 10.00 ± 0.05ab | 10.00 ± 0.03ab | - | 09.00 ± 0.03a | - | - | - |
| Aspergillus. niger KCTC 6317 | 09.00 ± 0.02a | 09.00 ± 0.01a | 10.00 ± 0.03ab | 09.00 ± 0.05a | - | - | - |
| List of Microorganisms | Heating at 95 °C for Different Times (min); Zone of Inhibition (mm) | ||
|---|---|---|---|
| 5 | 45 | 90 | |
| Gram-negative bacteria | |||
| S. enterica typhimurium ATCC 14,028 | 10.00 ± 0.02a | 13.00 ± 0.01b | 15.00 ± 0.03c |
| E. coli ATCC 35,150 | 09.00 ± 0.01a | 12.00 ± 0.02b | 09.00 ± 0.01a |
| E. coli ATCC 43,894 | 10.00 ± 0.02a | 10.00 ± 0.02a | 12.00 ± 0.03b |
| Gram-positive bacteria | |||
| S. aureus ATCC 13,150 | 10.00 ± 0.02a | 14.50 ± 0.01b | 15.50 ± 0.03bc |
| S. aureus ATCC 12,600 | 10.00 ± 0.02a | 12.00 ± 0.02b | 12.00 ± 0.03b |
| L. monocytogenes ATCC 19,118 | 11.00 ± 0.03a | 15.00 ± 0.02b | 16.30 ± 0.05 c |
| B. cereus ATCC 14,579 | 12.00 ± 0.04a | 15.00 ± 0.02b | 20.00 ± 0.03c |
| Fungi | |||
| C. albicans KCTC 7965 | 08.00 ± 0.02a | 10.50 ± 0.03b | 14.00 ± 0.01c |
| A. fumigatus KCTC 6145 | 08.50 ± 0.01a | 11.00 ± 0.05b | 11.00 ± 0.03b |
| A. flavus var. flavus KCTC 6143 | 10.00 ± 0.03a | 11.30 ± 0.02b | 13.00 ± 0.01c |
| A. niger KCTC 6317 | 09.00 ± 0.01a | 10.00 ± 0.04ab | 12.30 ± 0.03c |
| Compound | Chemical Formula | Area (%) | Molecular Weight (g.mol−1) | Docking Score (Kcal.mol−1) | Activity | References | |
|---|---|---|---|---|---|---|---|
| 4PLB | LpxC | ||||||
| Methylsulfonylmethane | C2H6O2S | 0.04 | 94.133 | −6.10 | −6.32 | Antioxidant, anti-inflammatory, anti-cancer | [26] |
| 2-Furancarboxaldehyde | C5H4O2 | 0.11 | 96.084 | −5.84 | −5.70 | Antibacterial | [27,28] |
| 5-Methylfuran-2-carbaldehyde | C6H6O2 | 0.05 | 110.111 | −6.94 | −6.96 | Pharmaceutical properties, organic inhibitor | [29] |
| 4H-Pyran-4-one | C5H4O2 | 0.06 | 96.084 | −5.40 | −5.91 | Pharmacological activity, | [28] |
| Benzofuran | C8H6O | 0.01 | 118.133 | −8.229 | −8.11 | Anti-inflammatory analgesic, antimicrobial | [30] |
| 2-Purinol | C5H4N4O | 0.04 | 136.111 | −6.14 | −6.04 | Antioxidant, potential of novel pharmaceuticals; anti-proliferative | [31] |
| 2-Methoxy-4-vinyphenol | C9H10O2 | 0.01 | 150.174 | −7.70 | −8.63 | Antimicrobial, antioxidant, anti-inflammatory, analgesic, anti-germination | [30] |
| Sr. No | Plant Extract | IC50 (µg.mL−1) MCF-7 |
|---|---|---|
| 1 | RC-Chloroform Extract | >50 |
| 2 | RC-Dichloromethane Extract | >50 |
| 3 | RC-Toluene Extract | >50 |
| 4 | RC-Ethyl ether Extract | >50 |
| 5 | RC-Ethanol Extract | >50 |
| 6 | RC-Methanol Extract | >50 |
| 7 | Tamoxifen | 10.08 |
| Quality Attributes | Storage Time (days) at 4 °C | |||||
|---|---|---|---|---|---|---|
| Treatments | 0 | 4 | 8 | 12 | 16 | |
| pH | Control | 5.64 ± 0.02a | 5.37 ± 0.01a | 6.29 ± 0.01a,b | 6.61 ± 0.01b | 6.89 ± 0.03b |
| RCC-A | 5.63 ± 0.01a | 5.31 ± 0.05a | 5.69 ± 0.05a | 5.61 ± 0.05a | 5.73 ± 0.03a | |
| RCC-B | 5.61 ± 0.01a | 5.23 ± 0.08a | 5.51 ± 0.08a | 5.53 ± 0.01a | 5.62 ± 0.05a | |
| TBARS (mg MDA/kg) | Control | 0.29 ± 0.01a | 0.99 ± 0.01a,b | 1.45 ± 0.01a,b | 1.84 ± 0.08a | 2.73 ± 0.04b |
| RCC-A | 0.25 ± 0.05a | 0.55 ± 0.01a | 0.98 ± 0.01a | 1.51 ± 0.01a | 2.05 ± 0.03a | |
| RCC-B | 0.26 ± 0.06a | 0.46 ± 0.09a | 0.89 ± 0.01a | 1.42 ± 0.08a | 1.62 ± 0.05a | |
| Moisture (%) | Control | 43.28 ± 0.03a | 41.07 ± 0.02b | 38.40 ± 0.02a | 36.05 ± 0.06a,b | 32.16 ± 0.08a |
| RCC-A | 43.17 ± 0.05a | 40.79 ± 0.01a | 39.14 ± 0.02a,b | 35.57 ± 0.07a | 32.33 ± 0.05a | |
| RCC-B | 43.31 ± 0.06a | 41.06 ± 0.04 b | 40.05 ± 0.03b | 37.21 ± 0.05b | 34.04 ± 0.07 b | |
| Treatments | Parameters | Storage Time (days) at 4 °C | ||||
|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | ||
| Control | L* | 48.44 ± 0.08a | 47.42 ± 0.02a | 44.12 ± 0.08a | 40.96 ± 0.06a | 36.72 ± 0.06a |
| RCC-A | 48.31 ± 0.05a | 47.00 ± 0.03a | 44.96 ± 0.04ab | 41.82 ± 0.09b | 37.66 ± 0.06b | |
| RCC-B | 48.26 ± 0.02a | 47.47 ± 0.08a | 45.54 ± 0.05b | 42.79 ± 0.03c | 39.99 ± 0.05c | |
| Control | a* | 15.41 ± 0.05a | 11.82 ± 0.02ab | 11.44 ± 0.02c | 10.33 ± 0.01c | 6.13 ± 0.03c |
| RCC-A | 15.65 ± 0.04a | 12.78 ± 0.03b | 7.45 ± 0.03a | 4.46 ± 0.02a | 2.93 ± 0.08a | |
| RCC-B | 15.20 ± 0.04a | 11.12 ± 0.04a | 8.63 ± 0.07b | 6.42 ± 0.02b | 4.76 ± 0.04b | |
| Control | b* | 6.48 ± 0.04a | 4.14 ± 0.05a | 2.43 ± 0.02a | 1.31 ± 0.08a | -0.43 ± 0.05a |
| RCC-A | 6.75 ± 0.04a | 5.54 ± 0.08b | 5.45 ± 0.07b | 2.81 ± 0.01b | 0.58 ± 0.06b | |
| RCC-B | 6.61 ± 0.02a | 5.89 ± 0.02b | 5.92± 0.03b | 4.69 ± 0.06c | 2.56 ± 0.02c | |
| Days | Treatments | Texture parameters | ||||
|---|---|---|---|---|---|---|
| Hardness (g) | Cohesiveness | Springiness (mm) | Chewiness (mJ) | Gumminess (g) | ||
| 0th | Control | 1160 ± 0.02a | 0.53 ± 0.03a | 1.61 ± 0.01a | 10.9 ± 0.05a | 583 ± 0.03a |
| RCC-A | 1190 ± 0.04a | 0.53 ± 0.01a | 1.53 ± 0.03a | 10.9 ± 0.06a | 590 ± 0.04a | |
| RCC-B | 1185 ± 0.05a | 0.56 ± 0.03a | 1.42 ± 0.03a | 10.4 ± 0.04a | 559 ± 0.05a | |
| 4th | Control | 810 ± 0.04a | 0.74 ± 0.02a | 1.78 ± 0.02a | 10.5 ± 0.03a | 599 ± 0.01a |
| RCC-A | 905 ± 0.01b | 0.53 ± 0.03a | 1.69 ± 0.04a | 12.00 ± 0.02b | 618 ± 0.03a | |
| RCC-B | 1000 ± 0.03c | 0.53 ± 0.03a | 1.73 ± 0.05a | 10.8 ± 0.02a | 636 ± 0.03a | |
| 8th | Control | 710 ± 0.03a | 0.56 ± 0.05a | 1.27 ± 0.01a | 6.30 ± 0.03a | 406 ± 0.05a |
| RCC-A | 805 ± 0.05b | 0.53 ± 0.04a | 1.13 ± 0.03a | 11.8 ± 0.05b | 561 ± 0.06b | |
| RCC-B | 855 ± 0.04b | 0.59 ± 0.05a | 1.48 ± 0.02a | 5.20 ± 0.03b | 557 ± 0.07b | |
| 12th | Control | 500 ± 0.06a | 0.97 ± 0.03a | 0.81 ± 0.04a | 5.00 ± 0.01b | 380 ± 0.03a |
| RCC-A | 600 ± 0.03b | 0.74 ± 0.01a | 1.45 ± 0.03a | 9.40 ± 0.04b | 400 ± 0.03a | |
| RCC-B | 720 ± 0.04c | 0.56 ± 0.03a | 0.57 ± 0.05a | 4.10 ± 0.03a | 463 ± 0.02ab | |
| 16th | Control | 395 ± 0.05a | 0.59 ± 0.02a | 2.93 ± 0.03a | 10.0 ± 0.01a | 248 ±0.04a |
| RCC-A | 420 ± 0.06a | 0.16 ± 0.06a | 2.26 ± 0.01a | 10.2 ± 0.06a | 260 ±0.05a | |
| RCC-B | 530 ± 0.03b | 0.32 ± 0.02a | 2.78 ± 0.05a | 18.8 ± 0.00b | 389 ± 0.03b | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubab, M.; Chelliah, R.; Saravanakumar, K.; Barathikannan, K.; Wei, S.; Kim, J.-R.; Yoo, D.; Wang, M.-H.; Oh, D.-H. Bioactive Potential of 2-Methoxy-4-vinylphenol and Benzofuran from Brassica oleracea L. var. capitate f, rubra (Red Cabbage) on Oxidative and Microbiological Stability of Beef Meat. Foods 2020, 9, 568. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9050568
Rubab M, Chelliah R, Saravanakumar K, Barathikannan K, Wei S, Kim J-R, Yoo D, Wang M-H, Oh D-H. Bioactive Potential of 2-Methoxy-4-vinylphenol and Benzofuran from Brassica oleracea L. var. capitate f, rubra (Red Cabbage) on Oxidative and Microbiological Stability of Beef Meat. Foods. 2020; 9(5):568. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9050568
Chicago/Turabian StyleRubab, Momna, Ramachandran Chelliah, Kandasamy Saravanakumar, Kaliyan Barathikannan, Shuai Wei, Jong-Rae Kim, Daesang Yoo, Myeong-Hyeon Wang, and Deog-Hwan Oh. 2020. "Bioactive Potential of 2-Methoxy-4-vinylphenol and Benzofuran from Brassica oleracea L. var. capitate f, rubra (Red Cabbage) on Oxidative and Microbiological Stability of Beef Meat" Foods 9, no. 5: 568. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9050568