Sustainable Removal of Ammonia from the Anaerobic Digester Supernatant Line Using a Prussian Blue Analogue (PBA) Composite Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Part 1: Characterization of the Adsorbing Material
2.1.1. Preparation of the Me-HCFs and Beads
2.1.2. Isotherms
2.1.3. Isotherm Adsorption Models
2.1.4. Morphology and Surface Area Characterization of the Me-HCF Powder and Beads
2.1.5. Ion Exchange Capacity and Cation Affinity Sequence
2.1.6. Simulation of a ZnHCF-Composite Bead Packed Column
2.2. Part 2: Selection of the CIX Working Conditions
2.2.1. Wastewater Solution
2.2.2. Column Experiments
2.2.3. Determination of the Required Hydraulic Retention Time
2.2.4. Reusing the Regeneration Solution—Full Adsorption/Regeneration Cycles
2.2.5. Analyses
3. Results and Discussion
3.1. Part 1: Characterization of the Adsorbing Material
3.1.1. Adsorption Isotherms
3.1.2. Characterization of the ZnHCF Beads
3.2. Part 2: Selecting the Working Conditions
3.2.1. Determining the Most Suitable Hydraulic Retention Time (HRT)
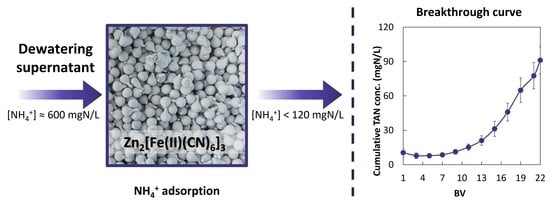
3.2.2. Breakthrough Curves and Regeneration Data
4. Cost Assessment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Ronan, E.; Aqeel, H.; Wolfaardt, G.M.; Liss, S.N. Recent Advancements in the Biological Treatment of High Strength Ammonia Wastewater. World J. Microbiol. Biotechnol. 2021, 37, 158. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Lei, Z.; Dzakpasu, M.; Li, Y.-Y.; Li, Q.; Chen, R. Application of the Anammox-Based Process for Nitrogen Removal from Anaerobic Digestion Effluent: A Review of Treatment Performance, Biochemical Reactions, and Impact Factors. J. Water Process Eng. 2020, 38, 101595. [Google Scholar] [CrossRef]
- Zhao, Q.; Peng, Y.; Li, J.; Gao, R.; Jia, T.; Deng, L.; Du, R. Sustainable Upgrading of Biological Municipal Wastewater Treatment Based on Anammox: From Microbial Understanding to Engineering Application. Sci. Total Environ. 2022, 813, 152468. [Google Scholar] [CrossRef]
- Zhao, J.; Zuo, J.; Lin, J.; Li, P. The Performance of a Combined Nitritation-Anammox Reactor Treating Anaerobic Digestion Supernatant under Various C/N Ratios. J. Environ. Sci. 2015, 30, 207–214. [Google Scholar] [CrossRef]
- Cao, S.; Yan, W.; Yu, L.; Zhang, L.; Lay, W.; Zhou, Y. Challenges of THP-AD Centrate Treatment Using Partial Nitritation-Anammox (PN/A)—Inhibition, Biomass Washout, Low Alkalinity, Recalcitrant and More. Water Res. 2021, 203, 117555. [Google Scholar] [CrossRef]
- Shih, K.; Yan, H. Chapter 26—The Crystallization of Struvite and Its Analog K-Struvite; From Waste Streams for Nutrient Recycling; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128038376. [Google Scholar]
- Wu, H.; Vaneeckhaute, C. Nutrient Recovery from Wastewater: A Review on the Integrated Physicochemical Technologies of Ammonia Stripping, Adsorption and Struvite Precipitation. Chem. Eng. J. 2022, 433, 133664. [Google Scholar] [CrossRef]
- Lahav, O.; Telzhensky, M.; Zewuhn, A.; Gendel, Y.; Gerth, J.; Calmano, W.; Birnhack, L. Struvite Recovery from Municipal-Wastewater Sludge Centrifuge Supernatant Using Seawater NF Concentrate as a Cheap Mg(II) Source. Sep. Purif. Technol. 2013, 108, 103–110. [Google Scholar] [CrossRef]
- Huang, K.-L. Removal of Organic and Ammonium Nitrogen Pollutants in Swine Wastewater Using Electrochemical Advanced Oxidation. Int. J. Electrochem. Sci. 2018, 13, 11418–11431. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Fujimoto, M.; Minami, K.; Takahashi, A.; Parajuli, D.; Hiwatari, T.; Kawakami, M.; Kawamoto, T. Ammonium Removal and Recovery from Sewage Water Using Column-System Packed Highly Selective Ammonium Adsorbent. Environ. Pollut. 2021, 284, 117495. [Google Scholar] [CrossRef]
- Thornton, A.; Pearce, P.; Parsons, S.A. Ammonium Removal from Digested Sludge Liquors Using Ion Exchange. Water Res. 2007, 41, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Lahav, O.; Schwartz, Y.; Nativ, P.; Gendel, Y. Sustainable Removal of Ammonia from Anaerobic-Lagoon Swine Waste Effluents Using an Electrochemically-Regenerated Ion Exchange Process. Chem. Eng. J. 2013, 218, 214–222. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Zielińska, M. Bacterial Communities in Full-Scale Wastewater Treatment Systems. World J. Microbiol. Biotechnol. 2016, 32, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miłobędzka, A.; Witeska, A.; Muszyński, A. Factors Affecting Population of Filamentous Bacteria in Wastewater Treatment Plants with Nutrients Removal. Water Sci. Technol. 2016, 73, 790–797. [Google Scholar] [CrossRef]
- Speirs, L.B.M.; Rice, D.T.F.; Petrovski, S.; Seviour, R.J. The Phylogeny, Biodiversity, and Ecology of the Chloroflexi in Activated Sludge. Front. Microbiol. 2019, 10, 02015. [Google Scholar] [CrossRef] [Green Version]
- Guida, S.; van Peteghem, L.; Luqmani, B.; Sakarika, M.; McLeod, A.; McAdam, E.J.; Jefferson, B.; Rabaey, K.; Soares, A. Ammonia Recovery from Brines Originating from a Municipal Wastewater Ion Exchange Process and Valorization of Recovered Nitrogen into Microbial Protein. Chem. Eng. J. 2022, 427, 130896. [Google Scholar] [CrossRef]
- Tanaka, J.; Matsumura, M. Application of Ozone Treatment for Ammonia Removal in Spent Brine. Adv. Environ. Res. 2003, 7, 835–845. [Google Scholar] [CrossRef]
- Gendel, Y.; Lahav, O. Revealing the Mechanism of Indirect Ammonia Electrooxidation. Electrochim. Acta 2012, 63, 209–219. [Google Scholar] [CrossRef]
- Ben-Asher, R.; Stefánsson, G.; Ólafsdóttir, A.; Mayer, H.; Nahir, R.; Gendel, Y.; Lahav, O. On-Board Zero-Discharge Water Treatment Unit for Well-Boats: Arctic Char as a Case Study. J. Appl. Aquac. 2022, 34, 953–968. [Google Scholar] [CrossRef]
- Lubensky, J.; Ellersdorfer, M. Pilot Scale Experiments for Ammonium Recovery from Sludge Liquor at a Municipal Waste Water Treatment Plant. J. Sustain. Dev. Energy Water Environ. Syst. 2021, 9, 1080349. [Google Scholar] [CrossRef]
- Lubensky, J.; Ellersdorfer, M.; Stocker, K. Ammonium Recovery from Model Solutions and Sludge Liquor with a Combined Ion Exchange and Air Stripping Process. J. Water Process Eng. 2019, 32, 100909. [Google Scholar] [CrossRef]
- Pesonen, J.; Myllymäki, P.; Tuomikoski, S.; Vervecken, G.; Hu, T.; Prokkola, H.; Tynjälä, P.; Lassi, U. Use of Calcined Dolomite as Chemical Precipitant in the Simultaneous Removal of Ammonium and Phosphate from Synthetic Wastewater and from Agricultural Sludge. ChemEngineering 2019, 3, 40. [Google Scholar] [CrossRef] [Green Version]
- Lahav, O.; Green, M. Ammonium Removal Using Ion Exchange and Biological Regeneration. Water Res. 1998, 32, 2019–2028. [Google Scholar] [CrossRef]
- Pabalan, R.T.; Bertetti, F.P. Cation-Exchange Properties of Natural Zeolites. Rev. Miner. Geochem. 2001, 45, 453–518. [Google Scholar] [CrossRef]
- Barrer, R.M.; Davies, J.A.; Rees, L.V.C. Thermodynamics and Thermochemistry of Cation Exchange in Chabazite. J. Inorg. Nucl. Chem. 1969, 31, 219–232. [Google Scholar] [CrossRef]
- Piernas Muñoz, M.J.; Castillo Martínez, E. Prussian Blue Based Batteries; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-91487-9. [Google Scholar]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Morphology-Dependent Electrochemical Performance of Zinc Hexacyanoferrate Cathode for Zinc-Ion Battery. Sci. Rep. 2015, 5, 18263. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhuang, S.; Liu, Y. Metal Hexacyanoferrates-Based Adsorbents for Cesium Removal. Coord Chem. Rev. 2018, 374, 430–438. [Google Scholar] [CrossRef]
- Naidu, G.; Nur, T.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Selective Sorption of Rubidium by Potassium Cobalt Hexacyanoferrate. Sep. Purif. Technol. 2016, 163, 238–246. [Google Scholar] [CrossRef]
- Jiang, Y.; Minami, K.; Sakurai, K.; Takahashi, A.; Parajuli, D.; Lei, Z.; Zhang, Z.; Kawamoto, T. High-Capacity and Selective Ammonium Removal from Water Using Sodium Cobalt Hexacyanoferrate. RSC Adv. 2018, 8, 34573–34581. [Google Scholar] [CrossRef] [Green Version]
- Shakir, K.; Sohsah, M.; Soliman, M. Removal of Cesium from Aqueous Solutions and Radioactive Waste Simulants by Coprecipitate Flotation. Sep. Purif. Technol. 2007, 54, 373–381. [Google Scholar] [CrossRef]
- Nativ, P.; Ben-Asher, R.; Fridman-Bishop, N.; Lahav, O. Synthesis and Characterization of Zinc-Hexacyanoferrate Composite Beads for Controlling the Ammonia Concentration in Low-Temperature Live Seafood Transports. Water Res. 2021, 203, 117551. [Google Scholar] [CrossRef] [PubMed]
- Metcalf & Eddy Inc. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Professional: New York, NY, USA, 2013. [Google Scholar]
- Britton, A.; Koch, F.A.; Mavinic, D.S.; Adnan, A.; Oldham, W.K.; Udala, B. Pilot-Scale Struvite Recovery from Anaerobic Digester Supernatant at an Enhanced Biological Phosphorus Removal Wastewater Treatment Plant. J. Environ. Eng. Sci. 2005, 4, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, A.; Kitajima, A.; Parajuli, D.; Hakuta, Y.; Tanaka, H.; Ohkoshi, S.; Kawamoto, T. Radioactive Cesium Removal from Ash-Washing Solution with High PH and High K+-Concentration Using Potassium Zinc Hexacyanoferrate. Chem. Eng. Res. Des. 2016, 109, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, C.P.; Machado, F.M. Part of the book series: Carbon Nanostructures (CARBON). Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Bergmann, C.P., Machado, F.M., Eds.; Carbon Nanostructures; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-18874-4. [Google Scholar]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1919, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Marei, S.A.; Basahel, S.N.; Rahmatallah, A.B. Ammonium Ion Exchange Equilibrium on Potassium Zinc Hexacyanoferrate/II/ K2Zn3[Fe/CN/6]2. J. Radioanal. Nucl. Chem. Lett. 1986, 104, 217–222. [Google Scholar] [CrossRef]
- Charlton, S.R.; Parkhurst, D.L. Modules Based on the Geochemical Model PHREEQC for Use in Scripting and Programming Languages. Comput. Geosci. 2011, 37, 1653–1663. [Google Scholar] [CrossRef]
- Willis, R.B.; Montgomery, M.E.; Allen, P.R. Improved Method for Manual, Colorimetric Determination of Total Kjeldahl Nitrogen Using Salicylate. J. Agric. Food Chem. 1996, 44, 1804–1807. [Google Scholar] [CrossRef]
- Vlasselaer, S.; D’Olieslager, W.; D’Hont, M. Caesium Ion Exchange Equilibrium on Potassium-Zinc-Hexacyanoferrate(II) K2Zn3(Fe(CN)6)2. J. Inorg. Nucl. Chem. 1976, 38, 327–330. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; ISBN 9781118131473. [Google Scholar]
- CHEMCAD, V8.1.0.16649 by Chemstations, Inc. Available online: https://www.chemstations.com (accessed on 24 November 2022).
- Gräber, Y.; Nativ, P.; Lahav, O. A Pre-Treatment Concept for Increasing the Recovery Ratio of Coastline BWRO Plants, While Providing Mg2+ in the Product Water. Desalination 2021, 515, 115202. [Google Scholar] [CrossRef]
- Electric Power Monthly—U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/electricity/monthly/epm_table_grapher.php?t=table_5_03 (accessed on 4 April 2021).
- Price of Industrial Salt in the United States from 2016 to 2021, by Type. Available online: https://0-www-Statista-Com.brum.beds.ac.uk/Statistics/916733/Us-Salt-Prices-by-Type/ (accessed on 24 November 2022).
- Nativ, P.; Gräber, Y.; Aviezer, Y.; Lahav, O. A Simple and Accurate Approach for Determining the VFA Concentration in Anaerobic Digestion Liquors, Relying on Two Titration Points and an External Inorganic Carbon Analysis. ChemEngineering 2021, 5, 15. [Google Scholar] [CrossRef]







| Method | Feasibility | Readiness Level | Source | ||
|---|---|---|---|---|---|
| Technological | Cost | Reliability | |||
| Anammox | ✓✓✓ | ✓ | ✓✓ | ✓✓✓ | [2,3] |
| CIX–Zeolite | ✓✓ | ✓✓✓ | ✓✓✓ | ✓✓ | [12,13,21,22] |
| CIX–CuHCF | ✓✓ | ✓✓✓ | ✓✓✓ | ✓✓ | [11] |
| Struvite precipitation | ✓✓✓ | ✓✓ | ✓✓✓ | ✓✓✓ | [7,8,23] |
| Current method | ✓✓✓ | ✓✓✓ | ✓✓✓ | ✓✓✓ | This paper |
| Cation | Synthetic WW 1 | Synthetic WW 2 | 1st Batch RWW | 2nd Batch RWW |
|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/L | |
| Na+ | 1000 | 185 | 230 | 245 |
| K+ | 0 | 184 | 157 | 138 |
| NH4+ | 600 | 600 | 542 | 566 |
| Ca2+ | 0 | 11 | 30 | 57 |
| Mg2+ | 0 | 35 | 41 | 29 |
| Background | Metal | qemax | KL | R2 |
|---|---|---|---|---|
| mgN/g | L/mg | - | ||
| DIW | Cobalt | 59.88 | 0.1170 | 0.989 |
| Nickel | 27.72 | 0.0251 | 0.942 | |
| Zinc | 28.52 | 0.3967 | 0.969 | |
| SW | Cobalt | 60.56 | 0.0140 | 0.954 |
| Nickel | 37.13 | 0.0229 | 0.942 | |
| Zinc | 26.47 | 0.0104 | 0.985 |
| Flow Rate | Adsorbed Mass of TAN in the Column | Operational Capacity |
|---|---|---|
| BV/h | mgN | % |
| ½ | 4904 ± 226 | 88.2% |
| 1 | 4931 ± 141 | 88.7% |
| 2 | 4594 ± 172 | 82.6% |
| 4 | 4531 ± 51 | 81.5% |
| 8 | 4245 ± 59 | 76.3% |
| Cycle no. | Synthetic Wastewater | 1st and 2nd Batch of Real WW | ||||||
|---|---|---|---|---|---|---|---|---|
| Adsorbed Mass | Desorbed Mass | Regeneration Efficiency | Feed Conc. | Adsorbed Mass | Desorbed Mass | Regeneration Efficiency | Feed Conc. | |
| mgN | mgN | % | mgN/L | mgN | mgN | % | mgN/L | |
| 1 | 4383 | 4279 | 98% | 605 | 4513 | 3983 | 88% | 581 |
| 2 | 4387 | 4381 | 100% | 606 | 4568 | 4055 | 89% | 581 |
| 3 | 4605 | 4586 | 100% | 602 | 4037 | 3817 | 95% | 517 |
| 4 | 4591 | 4361 | 95% | 603 | 3956 | 3719 | 94% | 518 |
| 5 | 4518 | 4280 | 95% | 610 | 4047 | 3741 | 92% | 535 |
| 6 | 4525 | 4302 | 95% | 621 | 3940 | 3762 | 95% | 534 |
| 7 | 4427 | 4347 | 98% | 604 | 3905 | 3937 | 101% | 529 |
| 8 | 4446 | 4457 | 100% | 603 | 4060 | 4096 | 101% | 538 |
| 9 | 4457 | 4731 | 106% | 603 | 4208 | 4078 | 97% | 568 |
| 10 | 4503 | 4402 | 98% | 603 | 4038 | 3955 | 98% | 554 |
| Average | 4484 | 4413 | 98% | 606 | 4127 | 3914 | 95% | 545 |
| STDV | 74 | 138 | 5 | 222 | 137 | 23 | ||
| Parameter | Unit | Method #1—Electrolysis | Method #2—Stripping | |
|---|---|---|---|---|
| OPEX | NaOH | $¢/m3 | 1.00 | 1.05 |
| NaCl | $¢/m3 | 0.19 | 0.19 | |
| ZnHCF | $¢/m3 | 0.24 | 0.24 | |
| Energy | $¢/m3 | 1.53 | 1.45 | |
| CAPEX | Columns | $¢/m3 | 0.13 | 0.13 |
| ZnHCF | $¢/m3 | 0.19 | 0.19 | |
| OPEX | $¢/m3 | 2.96 | 2.94 | |
| CAPEX | $¢/m3 | 0.32 | 0.32 | |
| Total cost | $¢/m3 | 3.29 | 3.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nativ, P.; Derbew, Z.A.; Dagan-Jaldety, C.; Aviezer, Y.; Ben-Asher, R.; Lahav, O. Sustainable Removal of Ammonia from the Anaerobic Digester Supernatant Line Using a Prussian Blue Analogue (PBA) Composite Adsorbent. ChemEngineering 2022, 6, 97. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering6060097
Nativ P, Derbew ZA, Dagan-Jaldety C, Aviezer Y, Ben-Asher R, Lahav O. Sustainable Removal of Ammonia from the Anaerobic Digester Supernatant Line Using a Prussian Blue Analogue (PBA) Composite Adsorbent. ChemEngineering. 2022; 6(6):97. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering6060097
Chicago/Turabian StyleNativ, Paz, Zenebu Abera Derbew, Chen Dagan-Jaldety, Yaron Aviezer, Raz Ben-Asher, and Ori Lahav. 2022. "Sustainable Removal of Ammonia from the Anaerobic Digester Supernatant Line Using a Prussian Blue Analogue (PBA) Composite Adsorbent" ChemEngineering 6, no. 6: 97. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering6060097