Biogenic Amines in Alcohol-Free Beverages
Abstract
:1. Introduction
2. Clinical and Toxicological Aspects
3. Biogenic Amine Regulation
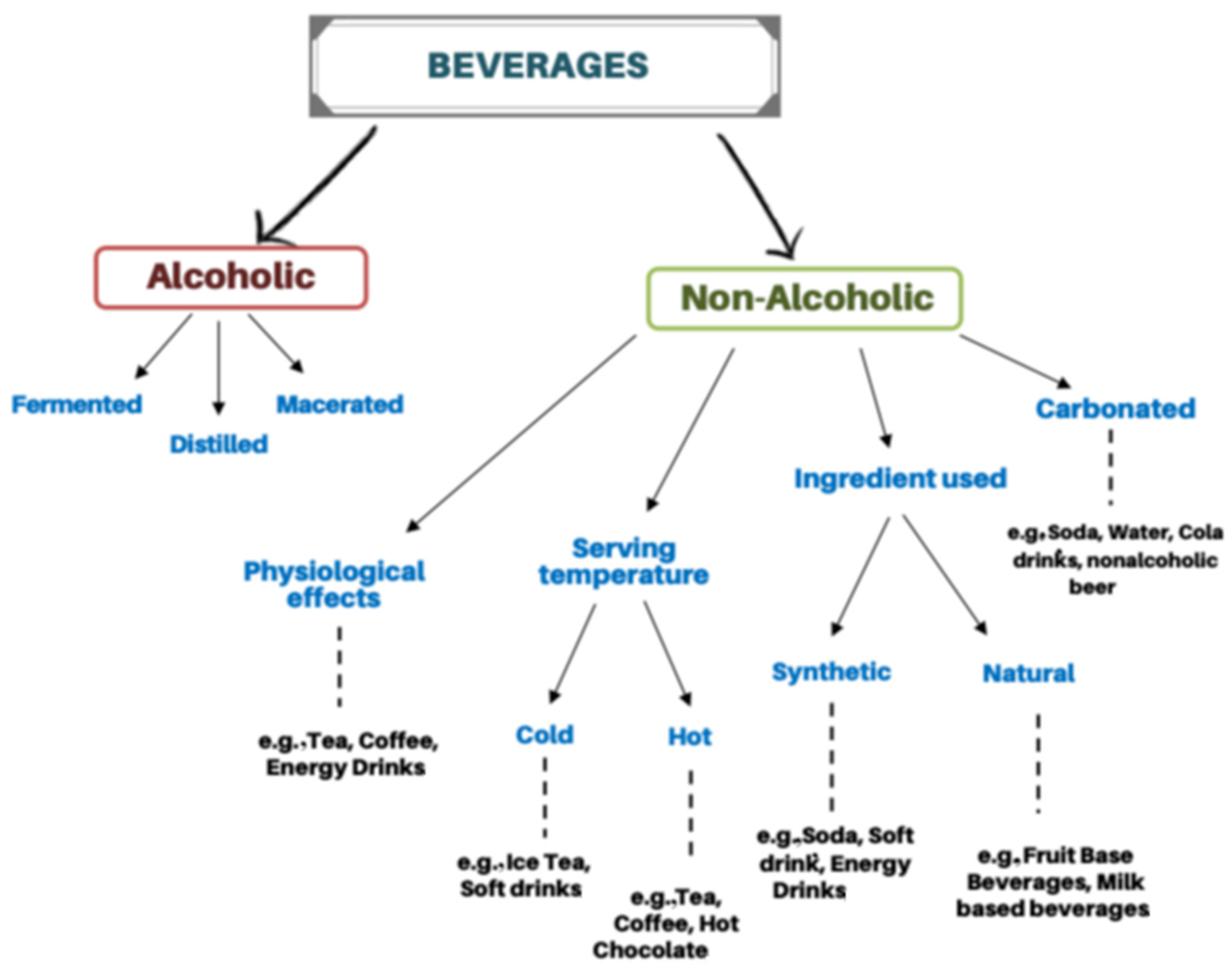
4. Beverage Classification
5. Biogenic Amines in Alcohol-Free and Non-Alcoholic Beverages
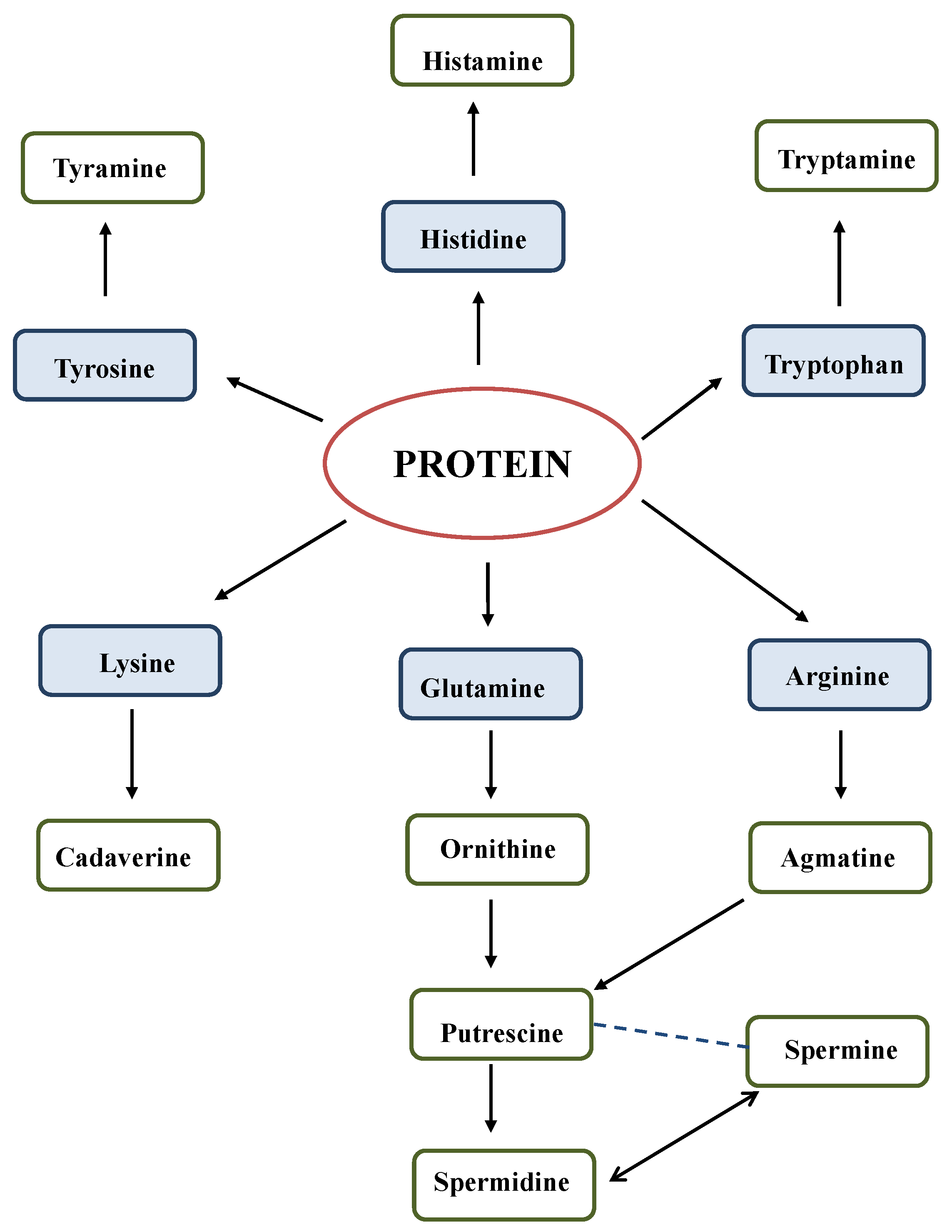
5.1. Biogenic Amine Synthesis in Alcohol-Free and Non-Alcoholic Beverages
5.2. Biogenic Amine Amount in “Plant Milk”
5.3. Biogenic Amine Amount in Fruit Juice
5.4. Biogenic Amine Content in Stimulating Beverages
5.5. Biogenic Amine Amount in Soft Drinks
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Him | Histamine |
| Tyr | Tyramine |
| Put | Putrescine |
| Cad | Cadaverine |
| Met | Methylamine |
| Agm | Agmatine |
| Phe | Phenylethylamine |
| Spm | Spermine |
| Spd | Spermidine |
| Try | Tryptamine |
| Ety | Ethylamine |
| Ser | Serotonin |
| nd | not detected |
| ni | not investigated |
| Bas | Biogenic amines |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
References
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B. Anthocyanins and anthocyanin-derived compounds. In Wine Chemistry and Biochemistry, 1st ed.; Victoria Moreno-Arribas, M., Carmen Polo, M., Eds.; Springer Science + Business Media, LLC: New York, NY, USA, 2009; pp. 439–462. [Google Scholar]
- Dvoryanchikv, G.; Tomchik, S.M.; Chaudhari, N. Biogenic amine synthesis and uptake in rodent taste buds. J. Comp. Neurol. 2007, 505, 302–3013. [Google Scholar] [CrossRef] [PubMed]
- Toro-Funes, N.; Bosch-Fusté, J.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Effect of ultra-high-pressure homogenization treatment on the bioactive compounds of soya milk. Food Chem. 2014, 152, 597–602. [Google Scholar] [CrossRef]
- Ali, M.A.; Poortvliet, E.; Strömberg, R.; Yngve, A. Polyamines in foods: Development of a food database. Food Nutr. Res. 2011, 55, 5572–5586. [Google Scholar]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Taylor, S.L.; Eitenmiller, R.R. Histamine food poisoning: Toxicology and clinical aspects. Crit. Rev. Toxicol. 1986, 17, 91–128. [Google Scholar] [CrossRef]
- Russo, P.; Capozzi, V.; Spano, G.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Metabolites of microbial origin with an impact on health: Ochratoxin A and biogenic amines. Front. Microbiol. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Costantini, A.; Vaudano, E.; Pulcini, L.; Carafa, T.; Garcia-Moruno, E. An Overview on Biogenic Amines in Wine. Beverages 2019, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- UNCTAD. Standard International Trade Classification Revision 3. Int. Trade Commod. Stat. 2010. [Google Scholar] [CrossRef]
- Centre, M.; Education, V. Study of Food Products and Beverages Industry. Methodol. Cent. Vocat. Educ. Train. 2008, 12, 226–233. [Google Scholar]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction—A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Neofotistos, A.D.G.; Tsagkaris, A.S.; Danezis, G.P.; Proestos, C. Emerging Trends in Biogenic Amines Analysis. In Biogenic Amines; IntechOpen: London, UK, 2019. [Google Scholar]
- Mohammed, G.I.; Bashammakh, A.S.; Alsibaai, A.A.; Alwael, H.; El-Shahawi, M.S. A critical overview on the chemistry, clean-up and recent advances in analysis of biogenic amines in foodstuffs. TrAC Trends Anal. Chem. 2016, 78, 84–94. [Google Scholar] [CrossRef]
- Bardócz, S. Polyamines in food and their consequences for food quality and human health. Trends Food Sci. Technol. 1995, 6, 341–346. [Google Scholar] [CrossRef]
- Comas-Basté, O.; Luz Latorre-Moratalla, M.; Sánchez-Pérez, S.; Teresa Veciana-Nogués, M.; del Carmen Vidal-Carou, M. Histamine and Other Biogenic Amines in Food. From Scombroid Poisoning to Histamine Intolerance. Biog. Amines 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Visciano, P.; Schirone, M.; Tofalo, R.; Suzzi, G. Histamine poisoning and control measures in fish and fishery products. Front. Microbiol. 2014, 5, 500. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.L.; Stratton Jayne, E.; Nordlee, J.A. Histamine Poisoning (Scombroid Fish Poisoning): An Allergy-Like Intoxication. J. Toxicol. Clin. Toxicol. 1989, 27, 45–51. [Google Scholar] [CrossRef]
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Histamine food poisoning. Handb. Exp. Pharmacol. 2017, 241, 217–235. [Google Scholar]
- Emborg, J.; Laursen, B.G.; Dalgaard, P. Significant histamine formation in tuna (Thunnus albacares) at 2 °C- Effect of vacuum- and modified atmosphere-packaging on psychrotolerant bacteria. Int. J. Food Microbiol. 2005, 101, 263–279. [Google Scholar] [CrossRef]
- Taylor, S.L.; Lieber, E.R. In vitro inhibition of rat intestinal histamine-metabolizing enzymes. Food Cosmet. Toxicol. 1979, 17, 237. [Google Scholar] [CrossRef]
- Brown, R.E.; Haas, H.L. On the mechanism of histaminergic inhibition of glutamate release in the rat dentate gyrus. J. Physiol. 1999, 515, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Lenis, Y.Y.; Elmetwally, M.A.; Maldonado-Estrada, J.G.; Bazer, F.W. Physiological importance of polyamines. Zygote 2017, 25, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Moinard, C.; Cynober, L.; de Bandt, J.P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 84–197. [Google Scholar] [CrossRef] [PubMed]
- Mietz, J.L.; Karmas, E. Polyamine and histamine content of rockfish, salmon, lobster, and shrimp as an indicator of decomposition. J. Assoc. Off. Anal. Chem. (USA) 1978, 61, 139–145. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Kovacova-Hanuskova, E.; Buday, T.; Gavliakova, S.; Plevkova, J. Histamine, histamine intoxication and intolerance. Allergol. Immunopathol. (Madr.) 2015, 43, 498–506. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Jimenez Colmenero, F. Biogenic amines in meat and meat products. Crit. Rev. Food Sci. Nutr. 2004, 44, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef]
- Fogel, W.A.; Lewinski, A.; Jochem, J. Histamine in food: Is there anything to worry about? Biochem. Soc. Trans. 2007, 35, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.O.; Srinivasa, T.K. Biogenic amines in seafood: A review. J. Food Sci. Technol. 2016, 53, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and membrane transporters. Amino Acids 2014, 46, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, V.; Masciari, P.; Pezzi, M.; Mola, A.; Tiburzi, S.P.; Zinzi, M.C.; Scozzafava, A.; Verre, M. Histamine Poisoning from Ingestion of Fish or Scombroid Syndrome. Case Rep. Emerg. Med. 2014, 2014, 482531. [Google Scholar] [CrossRef]
- Stratta, P.; Badino, G. Scombroid poisoning. Can. Med Assoc. J. 2012, 184, 674. [Google Scholar] [CrossRef] [Green Version]
- Tofalo, R.; Perpetuini, G.; Schirone, M.; Suzzi, G. Biogenic Amines: Toxicology and Health Effect. Encycl. Food Health 2015, 71, 424–429. [Google Scholar]
- Shalaby, A.R. Changes in biogenic amines in mature and germinating legume seeds and their behavior during cooking. Mol. Nutr. Food Res. 2000, 44, 23–27. [Google Scholar] [CrossRef]
- Dickinson, G. Scombroid fish poisoning syndrome. Ann. Emerg. Med. 1982, 11, 487–489. [Google Scholar] [CrossRef]
- Marcobal, A.; de las Rivas, B.; Landete, J.M.; Tabera, L.; Muñoz, R. Tyramine and Phenylethylamine Biosynthesis by Food Bacteria. Crit. Rev. Food Sci. Nutr. 2012, 52, 448–467. [Google Scholar] [CrossRef] [Green Version]
- Ladero, V.; Calles-Enriquez, M.; Fernandez, M.A.; Alvarez, M. Toxicological Effects of Dietary Biogenic Amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Fankhauser, C.; Charieras, T.; Caille, D.; Rovei, V. Interaction of MAO inhibitors and dietary tyramine: A new experimental model in the conscious rat. J. Pharmacol. Toxicol. Methods 1994, 32, 219–224. [Google Scholar] [CrossRef]
- Medina, M.Á.; Urdiales, J.L.; Rodríguez-Caso, C.; Ramírez, F.J.; Sánchez-Jiménez, F. Biogenic amines and polyamines: Similar biochemistry for different physiological missions and biomedical applications. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 23–59. [Google Scholar] [CrossRef]
- Broadley, K.J. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010, 125, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P. Health effects and occurrence of dietary polyamines: A review for the period 2005-mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef]
- Eliassen, K.A.; Reistad, R.; Risoen, U.; Ronning, H.F. ScienceDirect -Food Chemistry: Dietary polyamines. Eur. J. Gastroenterol. Hepatol. 2002, 13, 273–280. [Google Scholar]
- Pegg, A.E. The function of spermine. IUBMB Life 2014, 66, 8–18. [Google Scholar] [CrossRef]
- FAO/WHO. Public Health Risks of Histamine and Other Biogenic Amines from Fish and Fishery Products; WHO (Word Health Organization): Geneva, Switzerland, 2013. [Google Scholar]
- EFSA. Scientific Opinion on risk-based control of biogenic amine formation in fermented foods. EFSA Eur. Food Saf. Auth. J. 2011, 9, 2393. [Google Scholar]
- Roger, S.; Tofalo, B. Biogenic Amines in Food: Analysis, Occurrence and Toxicity. Royal Society of Chemistry. Food Chem. Funct. Anal. 2019, 20, 2019. [Google Scholar]
- EU. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Union (EU): Brussels, Belgium, 2005. [Google Scholar]
- USFDA. Fish and Fishery Products Hazards and Controls Guidance; Department of Health and Human Services. Food and Drug Administration, Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2019.
- FSANZ. Imported Food Risk Statement: Fish and Fish Products from the Families Specified and Histamine; Food Safety Australia New Zealand: Canberra, Australia, 2016.
- Mohedano, M.L.; López, P.; Spano, G.; Russo, P. Controlling the formation of biogenic amines in fermented foods. In: Advances in Fermented Foods and Beverages: Improving Quality. Technol. Health Benefits 2015, 64, S95–S100. [Google Scholar]
- Bhattacharjee, C.; Saxena, V.K.; Dutta, S. Fruit juice processing using membrane technology: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 136–153. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Beverages: Processing and Technology; Scientific Publishers: Jodhpur, India, 2018. [Google Scholar]
- Kalac, P.; Krizek, M. A Review of Biogenic Amines and Polyamines in Beer. J. Inst. Brew. 2003, 109, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Brányik, T.; Vicente, A.A.; Dostálek, P.; Teixeira, J.A. A review of flavour formation in continuous beer fermentations. J. Inst. Brew. 2008, 114, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Montanari, L.; Floridi, S.; Marconi, O.; Tironzelli, M.; Fantozzi, P. Effect of mashing procedures on brewing. Eur. Food Res. Technol. 2005, 221, 175–179. [Google Scholar] [CrossRef]
- Lodolo, E.J.; Kock, J.L.F.; Axcell, B.C.; Brooks, M. The yeast Saccharomyces cerevisiae—The main character in beer brewing. FEMS Yeast Res. 2008, 8, 1018–1036. [Google Scholar] [CrossRef] [Green Version]
- EU. European Parliament and Council (EU) 2019/787 Regarding the Definition, Designation, Presentation and Labeling of Beverages Witty, Using the Names of Spirit Drinks in Presentation and Labeling of Other Foodstuffs, as well as the Protection of Geographica; European Union: Brussels, Belgium, 2019. [Google Scholar]
- Brereton, P.; Hasnip, S.; Bertrand, A.; Wittkowski, R.; Guillou, C. Analytical methods for the determination of spirit drinks. TrAC Trends Anal. Chem. 2003, 22, 19–25. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Flick, G.J.; Ankenman Granata, L. Biogenic amines in foods. Toxins Food 2004, 8, 121–153. [Google Scholar]
- FAO/WHO Commission. Discussion Paper Histamine, Codex Committee on Fish and Fishery Products, Joint FAO/WHO food Standards Programme; WHO (Word Health Organization): Geneva, Switzerland, 2012; Volume 7. [Google Scholar]
- Dadáková, E.; Pelikánová, T.; Kalač, P. Content of biogenic amines and polyamines in some species of European wild-growing edible mushrooms. Eur. Food Res. Technol. 2009, 230, 163–171. [Google Scholar]
- Linares, D.M.; Martín, M.C.; Ladero, V.; Álvarez, M.A.; Fernández, M. Biogenic amines in dairy products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef]
- Vinci, G.; Antonelli, M.L. Biogenic amines: Quality index of freshness in red and white meat. Food Control 2002, 13, 519–524. [Google Scholar] [CrossRef]
- Torracca, B.; Pedonese, F.; López, M.B.; Turchi, B.; Fratini, F.; Nuvoloni, R. Effect of milk pasteurisation and of ripening in a cave on biogenic amine content and sensory properties of a pecorino cheese. Int. Dairy J. 2016, 61, 189–195. [Google Scholar] [CrossRef]
- Casal, S.; Oliveira, M.B.P.P.; Ferreira, M.A. Determination of biogenic amines in coffee by an optimized liquid chromatographic method. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 2535–2549. [Google Scholar] [CrossRef]
- Wheaton, T.A.; Stewart, I. Quantitative analysis of phenolic amines using ion-exchange chromatography. Anal. Biochem. 1965, 12, 585–592. [Google Scholar] [CrossRef]
- Bouchereau, A.; Guenot, P.; Larher, F. Analysis of amines in plant materials. J. Chromatogr. B Biomed. Sci. Appl. 2000, 747, 49–67. [Google Scholar] [CrossRef]
- Sagratini, G.; Fernández-Franzón, M.; De Berardinis, F.; Font, G.; Vittori, S.; Mañes, J. Simultaneous determination of eight underivatised biogenic amines in fish by solid phase extraction and liquid chromatography-tandem mass spectrometry. Food Chem. 2012, 132, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Tassoni, A.; Tango, N.; Ferri, M. Comparison of biogenic amine and polyphenol profiles of grape berries and wines obtained following conventional, organic and biodynamic agricultural and oenological practices. Food Chem. 2013, 139, 405–413. [Google Scholar] [CrossRef]
- Linares, D.M.; Del Río, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C.; Alvarez, M.A. Factors influencing biogenic amines accumulation in dairy products. Front. Microbiol. 2012, 28, 180. [Google Scholar] [CrossRef] [Green Version]
- Calzada, J.; Del Olmo, A.; Picon, A.; Gaya, P.; Nuñez, M. Proteolysis and biogenic amine buildup in high-pressure treated ovine milk blue-veined cheese. J. Dairy Sci. 2013, 96, 4816–4829. [Google Scholar] [CrossRef]
- Sabaté, J.; Pujolà, M.; Labanda, J.; Llorens, J. Influence of pH and operation variables on biogenic amines nanofiltration. Sep. Purif. Technol. 2008, 58, 424–428. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kima, Y.G.; Her, J.Y.; Kim, M.K.; Lee, K.G. Reduction of biogenic amine contents in fermented soybean paste using food additives. LWT 2018, 98, 470–476. [Google Scholar] [CrossRef]
- Vieira, S.M.; Theodoro, K.H.; Glória, M.B.A. Profile and levels of bioactive amines in orange juice and orangesoft drink. Food Chem. 2007, 100, 895–903. [Google Scholar] [CrossRef]
- Krause, I.; Bockhardt, A.; Klostermeyer, H. Characterization of cheese ripening by free amino acids and biogenic amines and influence of bactofugation and heat-treatment of milk. Lait 1997, 77, 101–108. [Google Scholar] [CrossRef]
- EU. Council Directive (EC) N° 2001/112 Fruit Juices and Other Similar Products Intended for Human Consumption; European Union: Brussels, Belgium, 2001. [Google Scholar]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2046–2068. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, J.E.; Fox, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Righetti, L.; Tassoni, A.; Bagni, N. Polyamines content in plant derived food: A comparison between soybean and Jerusalem artichoke. Food Chem. 2008, 111, 852–856. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. TrAC Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, L.; Ciano, S.; Rapa, M.; Ruggieri, R. Biogenic Amines Determination in “Plant Milks”. Beverages 2019, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Echavarría, A.P.; Torras, C.; Pagán, J.; Ibarz, A. Fruit Juice Processing and Membrane Technology Application. Food Eng. Rev. 2011, 3, 136–158. [Google Scholar] [CrossRef]
- Preti, R.; Bernacchia, R.; Vinci, G. Chemometric evaluation of biogenic amines in commercial fruit juices. Eur. Food Res. Technol. 2016, 242, 2031–2039. [Google Scholar] [CrossRef]
- Preti, R.; Antonelli, M.L.; Bernacchia, R.; Vinci, G. Fast determination of biogenic amines in beverages by a core-shell particle column. Food Chem. 2015, 187, 555–562. [Google Scholar] [CrossRef]
- Basheer, C.; Wong, W.; Makahleh, A.; Tameem, A.A.; Salhin, A.; Saad, B.; Lee, H.K. Hydrazone-based ligands for micro-solid phase extraction-high performance liquid chromatographic determination of biogenic amines in orange juice. J. Chromatogr. A 2011, 1218, 4332–4339. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Gupta, M.; Verma, K.K. Salting-out assisted liquid-liquid extraction for the determination of biogenic amines in fruit juices and alcoholic beverages after derivatization with 1-naphthylisothiocyanate and high-performance liquid chromatography. J Chromatogr. A 2015, 1422, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Saaid, M.; Saad, B.; Ali, A.S.M. In situ derivatization hollow fibre liquid-phase microextraction for the determination of biogenic amines in food samples. J. Chromatogr. A 2009, 1216, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Arlorio, M.; Coïsson, J.D.; Martelli, A. Validation of an isocratic HPLC method based on the use of ABZ+ Plus phase for the simultaneous determination of methylxantines, chlorogenic acid, some hydroxy-benzoic and hydroxy-cinnamic acids. Application to cocoa, coffee, tea and cola-drinks. Chromatographia 2000, 52, 579–583. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Brückner, H.; Flassig, S.; Kirschbaum, J. Determination of biogenic amines in infusions of tea (Camellia sinensis) by HPLC after derivatization with 9-fluorenylmethoxycarbonyl chloride (Fmoc-Cl). Amino Acids 2012, 42, 877–885. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Picci, N.; Restuccia, D. Extraction efficiency of different solvents and LC-UV determination of biogenic amines in tea leaves and infusions. J. Anal. Methods Chem. 2016. [Google Scholar] [CrossRef] [Green Version]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Picci, N. Brewing effect on levels of biogenic amines in different coffee samples as determined by LC-UV. Food Chem. 2014, 11, 134. [Google Scholar] [CrossRef]
- Özdestan, Ö. Evaluation of bioactive amine and mineral levels in Turkish coffee. Food Res. Int. 2014, 61, 167–175. [Google Scholar] [CrossRef]
- Da Silveira, T.M.L.; Tavares, É.; Glória, M.B.A. Profile and levels of bioactive amines in instant coffee. J. Food Compos. Anal. 2007, 20, 451–457. [Google Scholar] [CrossRef]
- Clarke, R.J.; Vitzthum, O.G. Coffee: Recent Developments; Blackwell Science: Hoboken, NJ, USA, 2008. [Google Scholar]
- Aflaki, F.; Ghoulipour, V.; Saemian, N.; Salahinejad, M. A simple method for benzoyl chloride derivatization of biogenic amines for high performance liquid chromatography. Anal. Methods 2014, 6, 1482–1487. [Google Scholar] [CrossRef]
- Aflaki, F.; Ghoulipour, V.; Saemian, N.; Sheibani, S. Biogenic Amine Contents in Non-alcoholic Beers: Screening and Optimization of Derivatization. Food Anal. Methods 2014, 7, 713–720. [Google Scholar] [CrossRef]
- Sun, J.; Guo, H.X.; Semin, D.; Cheetham, J. Direct separation and detection of biogenic amines by ion-pair liquid chromatography with chemiluminescent nitrogen detector. J. Chromatogr. A 2011, 1218, 4689–4697. [Google Scholar] [CrossRef] [PubMed]
- Lavizzari, T.; Teresa Veciana-Nogués, M.; Bover-Cid, S.; Mariné-Font, A.; Carmen Vidal-Carou, M. Improved method for the determination of biogenic amines and polyamines in vegetable products by ion-pair high-performance liquid chromatography. J. Chromatogr. A 2006, 1129, 67–72. [Google Scholar] [CrossRef]
- Roll, R. Orange juice and weather. Am. Econ. Rev. 1984, 74, 861–880. [Google Scholar]
- Catarino, M.; Mendes, A. Non-alcoholic beer -A new industrial process. Sep. Purif. Technol. 2011, 79, 342–351. [Google Scholar] [CrossRef]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.M.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Coton, M.; Bach, B.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 3, S95–S100. [Google Scholar] [CrossRef] [Green Version]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef]
- Attila, S.; Çakir, B. Energy-drink consumption in college students and associated factors. Nutrition 2011, 27, 316–322. [Google Scholar] [CrossRef]
| Biogenic Amine | Physiological Effect | Reference |
|---|---|---|
| Histamine | Release of adrenaline and noradrenaline Allergic processes Stimulation of the smooth muscles of the uterus, intestine, and respiratory tract Stimulation of sensory and motor neurons Control of gastric secretion | [35,36,37,38,39] |
| Tyramine | Peripheral vascularization Increase in cardiac output Increased lacrimation and salivation Increased breathing Increased blood sugar levels Noradrenaline release of the sympathetic nervous system Migraine | [16,37,40,41] |
| Putrescine and cadaverine | Hypotension Bradycardia Lockjaw Extremity paralysis Enhancement of the toxicity of other amines | [37,42,43] |
| β-Phenethylamine | Noradrenaline release of the sympathetic nervous Increase in blood pressure Migraine | [37] |
| Tryptamine | Increase in blood pressure | [38] |
| Spermine and spermidine | Hypotension Bradycardia Enhancement of the toxicity of other amines | [38,39,40,41,42,43,44,45,46,47,48] |
| Regulation | Rroducts | Limit (mg/kg) | Reference |
|---|---|---|---|
| Reg. (EC) no. 1019/2013 (modify Reg. (EC) no. 2073/2005) | Fish species (Scombridae, Clupeidae, Engraulidae, Coryphenidae, Pomatomidae, and Scombreresosidae) | 100–200 | [51] |
| Fish products that have undergone enzyme maturation treatment | 200–400 | ||
| USFDA | Fish products | 500 | [52] |
| FSANZ | Sample composed of different species of fish | 100 | [53] |
| Codex Alimentarius | Fish and fish product (Clupeidae, Scrombridae, Scromberesocidae, Pomatomidae, and Coryphaenidae families) | 100 | [54] |
| Beverages | No. * | Biogenic Amine Amount (mg/L) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phe | Put | Cad | His | Tyr | Try | Spd | Spm | |||
| Soymilk-based product | 1 | ni * | ni | ni | ni | ni | ni | 8.18 | 1.98 | [4] |
| Soymilk UHT | 6 | ni | ni | ni | ni | ni | ni | 7.77 ÷ 8.37 | 1.91 ÷ 2.04 | |
| Spelt milk | 3 | nd * | nd | 0.49 ÷ 1.17 | 5.26 ÷ 6.64 | nd | ni | nd | nd | [87] |
| Oat milk | 3 | nd | nd | 0.64 ÷ 0.71 | 6.59 ÷ 8.06 | nd | ni | 0.46 ÷ 0.69 | 0.10 ÷ 0.17 | |
| Millet milk | 5 | nd | nd | nd ÷ 0.23 | 6.14 ÷ 8.37 | nd ÷ 0.25 | ni | nd | nd | |
| Barley milk | 4 | nd | nd | 1.47 ÷ 5.36 | nd ÷ 3.15 | 0.28 ÷ 0.62 | ni | nd ÷ 0.31 | nd ÷ 0.14 | |
| Quinoa milk | 4 | nd | nd | 0.52 ÷ 0.68 | 6.77 ÷ 7.69 | 0.10 ÷ 0.22 | ni | nd | nd ÷ 0.14 | |
| Rice milk | 4 | nd | nd | nd ÷ 0.25 | 3.10 ÷ 7.44 | nd | ni | - | nd ÷ 0.17 | |
| Beverages | No. * | Biogenic Amine Amount (mg/L) | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phe | Put | Cad | His | Tyr | Try | Spd | Spm | Ser | Ety | Met | |||
| Pear nectar | 3 | nd * | 1.23 ÷ 3.10 | 5.88 ÷ 17.2 | nd | nd | ni * | 1.70 ÷ 2.17 | 1.47 ÷ 2.19 | nd | nd | nd | [90] |
| Apricot nectar | 3 | nd | 1.10 ÷ 3.25 | 6.81 ÷ 11.25 | nd | nd | ni | 1.32 ÷ 2.95 | 2.21 ÷ 2.74 | nd | nd ÷ 2.45 | nd | |
| Peach nectar | 3 | nd | 1.88 ÷ 7.22 | 6.51 ÷ 13.03 | nd | nd | ni | 1.34 ÷ 1.96 | 1.58 ÷ 3.58 | nd | nd | nd | |
| Orange juice | 1 | 0.63 | 0.65 | 0.99 | 0.50 | 0.67 | 0.89 | 0.76 | ni | ni | ni | ni | [91] |
| Orange juice | 21 | nd | 22.6 ÷ 43.7 | nd | 0.03 ÷ 0.26 | 0.02 ÷ 0.67 | nd | 1.80 ÷ 4.20 | 0.08 ÷ 0.34 | nd ÷ 0.48 | ni | ni | [79] |
| Orange juice | 1 | 0.01 | 0.01 | ni | ni | nd | 0.05 | 0.01 | ni | ni | ni | 0.06 | [92] |
| Apple juice | 1 | 0.09 | 0.01 | ni | ni | 0.12 | 0.06 | 0.01 | ni | ni | ni | 0.78 | |
| Grape juice | 1 | 0.10 | 0.06 | ni | ni | nd | 0.35 | nd | ni | ni | ni | nd | |
| Mango juice | 1 | 0.17 | 0.06 | ni | ni | 0.14 | 0.31 | 0.01 | ni | ni | ni | nd | |
| Pineapple juice | 1 | 0.03 | 0.12 | ni | ni | 0.01 | 0.01 | nd | ni | ni | ni | 0.84 | |
| Litchi juice | 1 | 0.01 | 0.01 | ni | ni | 0.02 | nd | 0.03 | ni | ni | ni | 0.07 | |
| Apricot juice | 7 | nd | 1.39 ÷ 7.10 | 3.96 ÷ 17.93 | nd | nd | ni | 1.97 ÷ 2.52 | 1.22 ÷ 2.51 | nd | nd | nd | [89] |
| Peach 50% juice | 6 | nd | 1.41 ÷ 3.19 | 1.95 ÷ 10.06 | nd | nd | ni | 1.34 ÷ 2.01 | 1.22 ÷ 2.72 | nd | nd | nd | |
| Peach 70% juice | 6 | nd | 2.25 ÷ 3.59 | 4.11 ÷ 6.05 | nd | nd | ni | 2.02 ÷ 4.44 | 1.37 ÷ 1.89 | nd | nd | nd | |
| Pear 50% juice | 6 | nd | 1.11 ÷ 2.68 | 1.91 ÷ 8.31 | nd | nd | ni | 1.16 ÷ 1.77 | 1.17 ÷ 3.53 | nd | nd | nd | |
| Pear 70% juice | 6 | nd | 1.42 ÷ 4.44 | 3.75 ÷ 6.15 | nd | nd | ni | 1.90 ÷ 2.65 | 1.22 ÷ 1.41 | nd | 1.12 ÷ 1.23 | nd | |
| Apple concentrate juice | 10 | nd | 0.59 ÷ 1.68 | 0.55 ÷ 4.27 | nd | nd | ni | 0.24 ÷ 0.67 | 0.24 ÷ 0.99 | nd | nd ÷ 0.41 | nd | |
| Pineapple concentrate juice | 10 | nd | 1.53 ÷ 1.98 | nd ÷ 3.14 | nd | nd | ni | 2.55 ÷ 5.41 | 1.53 ÷ 3.17 | nd ÷ 4.61 | 0.22 ÷ 1.65 | nd ÷ 1.88 | |
| Grapefruit concentrate juice | 11 | nd | 7.17 ÷ 20.8 | 0.38 ÷ 2.28 | nd | nd | ni | 1.03 ÷ 2.11 | 0.32 ÷ 0.50 | nd ÷ 1.74 | 6.21 ÷ 12.98 | nd ÷ 1.17 | |
| Orange concentrate juice | 12 | nd | 34.70 ÷ 60.97 | nd | nd | nd | ni | 2.04 ÷ 3.66 | 0.37 ÷ 1.37 | nd ÷ 1.32 | 24.06 ÷ 38.64 | nd ÷ 2.72 | |
| Orange juice | 5 | nd | 0.55 ÷ 2.21 | ni | nd ÷ 0.04 | nd ÷ 0.06 | nd | 0.08 ÷ 0.14 | ni | ni | ni | ni | [91] |
| Beverages | No. * | Biogenic Amine Amount | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phe | Put | Cad | His | Tyr | Try | Agm | Spd | Spm | Ser | |||
| Black tea | 14 | nd * ÷ 0.03 | nd ÷ 0.02 | nd | nd | nd ÷ 0.19 | ni * | ni | nd ÷ 0.05 | nd ÷ 0.34 | ni | [96] |
| Green tea | 6 | nd | 0.02 ÷ 0.08 | nd | nd | nd ÷ 0.04 | ni | ni | 0.03 ÷ 0.09 | 0.06 ÷ 0.32 | ni | |
| Instant tea | 1 | 0.02 | nd | nd | nd | 0.03 | ni | ni | 0.05 | ni | ni | |
| Black tea infusion | 14 | nd ÷ 2.00 | 8.4 ÷ 10.2 | nd ÷ 14.0 | nd ÷ 20.0 | nd | ni | ni | 6.5 ÷ 10.8 | nd ÷ 0.3 | nd ÷ 14.1 | [97] |
| Green tea | 7 | nd | 10.3 ÷ 14.6 | nd | nd | nd | ni | ni | 6.3 ÷ 10.4 | nd | nd ÷ 11.5 | |
| Tea drinks | 3 | nd | nd ÷ 6.9 | nd | nd | nd | ni | ni | 4.3 ÷ 6.7 | nd | nd | |
| Espresso coffee | 20 | 0.20 ÷ 1.21 | 0.60 ÷ 2.27 | 0.19 ÷ 1.84 | 0.22 ÷ 1.62 | 0.25 ÷ 1.89 | ni | ni | 0.45 ÷ 1.20 | nd ÷ 1.95 | nd ÷ 0.90 | [98] |
| Turkish coffee | 10 | nd ÷ 4.99 | 0.5 ÷ 1.5 | 1.4 ÷ 9.0 | nd | nd ÷ 19.7 | ni | ni | nd | nd | 3.70 ÷ 13.55 | [99] |
| Instant coffee | 16 | 0.4 ÷ 7.4 | 0.4 ÷ 5.3 | 0.4 ÷ 8.1 | 0.4 ÷ 1.4 | 0.4 ÷ 5.5 | nd | 0.4 ÷ 5.3 | 0.4 ÷ 7.7 | 0.5 ÷ 4.1 | 8.00 ÷ 18.0 | [100] |
| Beverages | No. * | Biogenic Amines amount (mg/L) | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phe | Put | Cad | His | Tyr | Try | Agm | Spd | Spm | Ser | |||
| Orange carbonated-based | 35 | nd * | 0.69 ÷ 5.14 | nd | 0.01 ÷ 0.03 | nd | nd | nd | 0.01 ÷ 0.32 | 0.01 ÷ 0.04 | nd | [79] |
| Non-alcoholic beer | 10 | nd ÷ 0.54 | 0.31 ÷ 1.43 | 0.11 ÷ 0.42 | nd ÷ 0.62 | nd ÷ 0.48 | 0.29 ÷ 2.56 | nd | 0.16 ÷ 0.81 | 0.13 ÷ 0.68 | ni * | [102] |
| Non-alcoholic beer | 5 | nd ÷ 0.48 | 0.56 ÷ 1.30 | 0.28 ÷ 0.56 | nd ÷ 0.37 | nd ÷ 0.27 | 0.28 ÷ 1.30 | nd | 0.35 ÷ 0.73 | 0.24 ÷ 0.72 | ni | [103] |
| Dairy beverages | 1 | nd | 3.2 | nd | 1.90 | nd | nd | 3.10 | nd | nd | ni | [104] |
| Milk chocolate | 8 | ni | nd ÷ 0.40 | ni | nd | nd ÷ 0.03 | nd ÷ 0.10 | ni | nd ÷ 1.00 | nd ÷ 2.00 | ni | [105] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinci, G.; Maddaloni, L. Biogenic Amines in Alcohol-Free Beverages. Beverages 2020, 6, 17. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages6010017
Vinci G, Maddaloni L. Biogenic Amines in Alcohol-Free Beverages. Beverages. 2020; 6(1):17. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages6010017
Chicago/Turabian StyleVinci, Giuliana, and Lucia Maddaloni. 2020. "Biogenic Amines in Alcohol-Free Beverages" Beverages 6, no. 1: 17. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages6010017





