Effect of Methyl Jasmonate and Methyl Jasmonate Plus Urea Foliar Applications on Wine Phenolic, Aromatic and Nitrogen Composition
Abstract
:1. Introduction
2. Materials and Methods
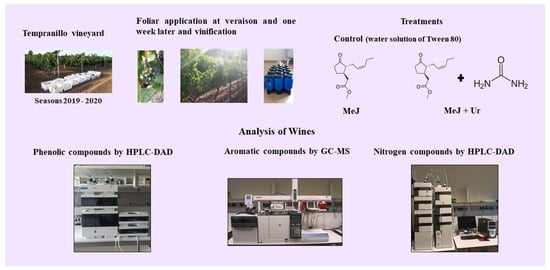
2.1. Vineyard Site, Grapevine Treatments and Vinification
2.2. Determination of Enological Parameters
2.3. Analysis of Wine Phenolic Compounds by HPLC-DAD
2.3.1. Sample Preparation for the Determination of Non-Anthocyanin Phenolic Compounds
2.3.2. Analysis of Phenolic Compounds by HPLC-DAD
2.4. Determination of Wine Aromatic Compounds by GC-MS
2.5. Analysis of Wine Nitrogen Compounds by HPLC-DAD
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of MeJ and MeJ+Ur Foliar Applications on Wine Enological Parameters
3.2. Influence of the Foliar MeJ and MeJ+Ur Treatments on Wine Phenolic Composition
3.3. Effect of the Foliar MeJ and MeJ+Ur Applications on Wine Aromatic Compounds
3.4. Influence of the Foliar MeJ and MeJ+Ur Treatments on Wine Nitrogen Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Portu, J.; López, R.; Santamaría, P. Garde-Cerdán, T. Methyl jasmonate treatment to increase grape and wine phenolic content in Tempranillo and Graciano varieties during two growing seasons. Sci. Hortic. 2018, 240, 378–386. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Freitas, V. Influence of phenolics on wine organoleptic properties. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 529–570. [Google Scholar]
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Chemical Composition and Polyphenolic Compounds of Red Wines: Their Antioxidant Activities and Effects on Human Health—A Review. Beverages 2022, 8, 1. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. LWT Food Sci. Technol. 2008, 41, 501–510. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; López, R.; Portu, J.; González-Arenzana, L.; López-Alfaro, I.; Santamaría, P. Study of the effects of proline, phenylalanine, and urea foliar application to Tempranillo vineyards on grape amino acid content. Comparison with commercial nitrogen fertilisers. Food Chem. 2014, 163, 136–141. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Li, J. Aroma Compounds in Wine. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Rubio-Bretón, P.; Marín-San Román, S.; Baroja, E.; Sáenz de Urturi, I.; Pérez-Álvarez, E.P. Study of wine volatile composition of Tempranillo versus Tempranillo Blanco, a new white grape variety. Beverages 2021, 7, 72. [Google Scholar] [CrossRef]
- Reynolds, A.G. Viticultural and vineyard management practices and their effects on grape and wine quality, 2nd ed.; In Food Science, Technology and Nutrition, Managing Wine Quality, Reynolds, A.G., Ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2022; pp. 443–539. ISBN 9780081020678. [Google Scholar]
- Pérez-Porras, P.; Bautista-Ortín, A.B.; Jurado, R.; Gómez-Plaza, E. Combining high-power ultrasound and enological enzymes during winemaking to improve the chromatic characteristics of red wine. LWT Food Sci. Technol. 2022, 156, 113032. [Google Scholar] [CrossRef]
- Gambacorta, G.; Antonacci, D.; Pati, S.; la Gatta, M.; Faccia, M.; Coletta, A.; La Notte, E. Influence of winemaking technologies on phenolic composition of Italian red wines. Eur. Food Res. Technol. 2011, 233, 1057–1066. [Google Scholar] [CrossRef]
- Delaunois, B.; Farace, G.; Jeandet, P.; Clément, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Elicitors as alternative strategy to pesticides in grapevine? Current knowledge on their mode of action from controlled conditions to vineyard. Environ. Sci. Pollut. Res. 2014, 21, 4837–4846. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López- Roca, J.M. Effect of benzothiadiazole and methyl jasmonate on the volatile compound composition of Vitis vinifera L. Monastrell grapes and wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Effect of methyl jasmonate on the aroma of Sangiovese grapes and wines. Food Chem. 2018, 242, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; Santamaría, P.; López-Alfaro, I.; López, R.; Garde-Cerdán, T. Methyl jasmonate foliar application to Tempranillo vineyard improved grape and wine phenolic content. J. Agric. Food Chem. 2015, 63, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; López-Alfaro, I.; Gómez-Alonso, S.; López, R.; Garde-Cerdán, T. Changes on grape phenolic composition induced by grapevine foliar applications of phenylalanine and urea. Food Chem. 2015, 180, 171–180. [Google Scholar] [CrossRef]
- Portu, J.; González-Arenzana, L.; Hermosín-Gutiérrez, I.; Santamaría, P.; Garde-Cerdán, T. Phenylalanine and urea foliar applications to grapevine: Effect on wine phenolic content. Food Chem. 2015, 180, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Portu, J.; Lopez, R.; Santamaría, P. Effect of foliar applications of proline, phenylalanine, urea, and commercial nitrogen fertilizers on stilbene concentration in Tempranillo musts and wines. Am. J. Enol. Vitic. 2015, 66, 4. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Fernández-Fernández, J.I.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.; Gómez-Martínez, J.C.; Martínez-Jiménez, J.A.; Gil-Muñoz, R. Application of elicitors in two ripening periods of Vitis vinifera L. cv Monastrell: Influence on anthocyanin concentration of grapes and wines. Molecules 2021, 26, 1689. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Paladines-Quezada, D.F. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 227, 691–697. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Ruiz-García, Y.; Williams, P.; Gil-Muñoz, R.; Gómez-Plaza, E.; Doco, T. Preharvest application of elicitors to Monastrell grapes: Impact on wine polysaccharide and oligosaccharide composition. J. Agric. Food Chem. 2018, 66, 11151–11157. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Effect of methyl jasmonate doped nanoparticles on nitrogen composition of Monastrell grapes and wines. Biomolecules 2021, 11, 1631. [Google Scholar] [CrossRef]
- Ancín-Azpilicueta, C.; Nieto-Rojo, R.; Gómez-Cordón, J. Effect of foliar urea fertilisation on volatile compounds in Tempranillo wine. J. Sci. Food Agric. 2013, 93, 1485–1491. [Google Scholar] [CrossRef]
- Rubio-Bretón, P.; Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.P.; Santamaría, P.; Garde-Cerdán, T. Foliar application of several nitrogen sources as fertilisers to Tempranillo grapevines: Effect on wine volatile composition. S. Afr. J. Enol. Vitic. 2018, 39, 2. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Portu, J.; López, R.; Santamaría, P. Effect of methyl jasmonate application to grapevine leaves on grape amino acid content. Food Chem. 2016, 203, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Gutiérrez-Gamboa, G.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of methyl jasmonate foliar application to vineyard on grape volatile composition over three consecutive vintages. Food Res. Int. 2018, 112, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, E.P.; Ramírez-Rodríguez, G.B.; Carmona, F.J.; Martínez-Vidaurre, J.M.; Masciocchi, N.; Guagliardi, A.; Garde-Cerdán, T.; Delgado-López, J.M. Towards a more sustainable viticulture: Foliar application of N-doped calcium phosphate nanoparticles on Tempranillo grapes. J. Sci. Food Agric. 2021, 101, 1307–1313. [Google Scholar] [CrossRef]
- OIV. Compendium of Internationals Methods of Wine and Must Analysis; OIV: Paris, France, 2009. [Google Scholar]
- Castillo-Muñoz, N.; Fernández-González, M.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. J. Agric. Food Chem. 2009, 57, 7883–7891. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Martínez-Gil, A.M.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Salinas, M.R. Implications of nitrogen compounds during alcoholic fermentation from some grape varieties at different maturation stages and cultivation systems. Food Chem. 2011, 124, 106–116. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Ancín-Azpilicueta, C.; Salinas, R. Study of the evolution of nitrogen compounds during grape ripening. Application to differentiate grape varieties and cultivated systems. J. Agric. Food Chem. 2009, 57, 2410–2419. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Methyl jasmonate effects on table grape ripening, vine yield, berry quality and bioactive compounds depend on applied concentration. Sci. Hortic. 2019, 247, 380–389. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Changes on grape volatile composition through elicitation with methyl jasmonate, chitosan, and a yeast extract in Tempranillo (Vitis vinifera L.). Sci. Hortic. 2019, 244, 257–262. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A tool for improving fruit phenolic content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. A first approach towards the relationship between grape skin cell-wall composition and anthocyanin extractability. Anal. Chim. Acta 2006, 563, 26–32. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving grape phenolic content and wine chromatic characteristics through the use of two different elicitors: Methyl jasmonate versus benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; Santamaría, P.; López, R.; Garde-Cerdán, T. Phenolic composition of Tempranillo grapes following foliar applications of phenylalanine and urea: A two-year study. Sci. Hortic. 2017, 219, 191–199. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Ewald, P.; Santamaría, P.; Winterhalter, P.; Garde-Cerdán, T. Evaluation of Grenache, Graciano and Tempranillo grape stilbene content after field applications of elicitors and nitrogen compounds. J. Sci. Food Agric. 2018, 98, 1856–1862. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; G-Alegría, E.; Polo, M.C.; Tenorio, C.; Martín-Álvarez, P.J.; Calvo De La Banda, M.T.; Ruiz-Larrea, F.; Moreno-Arribas, M.V. Wine volatile and amino acid composition after malolactic fermentation: Effect of Oenococcus oeni and Lactobacillus plantarum starter cultures. J. Agric. Food Chem. 2005, 53, 8729–8735. [Google Scholar] [CrossRef]
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]


| 2019 | 2020 | |||||
|---|---|---|---|---|---|---|
| Control | MeJ | MeJ+Ur | Control | MeJ | MeJ+Ur | |
| Alcohol (% v/v) | 13.97 ± 0.31 b | 12.57 ± 0.25 a | 12.80 ± 0.40 a | 12.47 ± 0.70 a | 12.18 ± 1.59 a | 12.53 ± 0.81 a |
| pH | 3.96 ± 0.07 a | 3.90 ± 0.10 a | 3.94 ± 0.13 a | 3.66 ± 0.08 a | 3.70 ± 0.04 a | 3.73 ± 0.13 a |
| Total acidity (g/L) * | 4.27 ± 0.10 b | 4.08 ± 0.06 a | 3.92 ± 0.06 a | 4.43 ± 0.59 a | 4.38 ± 0.23 a | 4.02 ± 0.23 a |
| V A 1 (g/L) ** | 0.23 ± 0.02 a | 0.28 ± 0.03 b | 0.20 ± 0.01 a | 0.22 ± 0.02 b | 0.18 ± 0.01 a | 0.22 ± 0.03 b |
| Lactic acid (g/L) | 1.32 ± 0.10 a | 1.36 ± 0.07 a | 1.28 ± 0.12 a | 0.86 ± 0.07 a | 1.14 ± 0.15 b | 1.05 ± 0.09 b |
| YAN 2 (mg N/L) | 18.06 ± 2.08 a | 41.65 ± 3.90 b | 67.89 ± 8.90 c | 30.36 ± 0.54 a | 28.40 ± 12.49 a | 39.34 ± 10.65 a |
| T P 3 (mg/L) | 2440.83 ± 123.16 a | 2160.37 ± 221.12 a | 2460.73 ± 124.74 a | 1116.63 ± 106.69 a | 1263.07 ± 224.95 a | 1333.47 ± 153.38 a |
| T A 4 (mg/L) | 1117.33 ± 69.97 a | 1225.67 ± 98.64 a | 1289.67 ± 102.00 a | 130.99 ± 20.13 a | 158.53 ± 18.35 a | 168.00 ± 18.68 a |
| CI 5 | 18.27 ± 1.03 a | 17.53 ± 1.81 a | 19.01 ± 1.14 a | 6.05 ± 0.55 a | 7.70 ± 2.13 a | 8.62 ± 1.10 a |
| TPI 6 | 70.83 ± 3.47 a | 66.43 ± 7.95 a | 73.32 ± 5.00 a | 36.82 ± 4.05 a | 41.04 ± 8.69 a | 44.73 ± 5.62 a |
| 2019 | 2020 | |||||
|---|---|---|---|---|---|---|
| Control | MeJ | MeJ+Ur | Control | MeJ | MeJ+Ur | |
| Delphinidin-3-glc | 14.67 ± 2.72 a | 17.06 ± 1.23 a | 15.68 ± 1.44 a | 6.48 ± 0.67 a | 11.03 ± 1.09 b | 10.20 ± 1.88 b |
| Cyanidin-3-glc | 2.21 ± 0.06 a | 2.44 ± 0.41 a | 2.54 ± 0.33 a | 1.57 ± 0.07 a | 1.78 ± 0.19 ab | 1.88 ± 0.13 b |
| Petunidin-3-glc | 20.48 ± 3.40 a | 22.94 ± 3.45 a | 22.68 ± 1.06 a | 13.81 ± 2.37 a | 18.22 ± 1.49 b | 17.45 ± 2.27 ab |
| Peonidin-3-glc | 6.38 ± 0.60 a | 9.43 ± 0.84 b | 8.70 ± 1.07 b | 2.83 ± 0.56 a | 4.11 ± 0.55 ab | 4.33 ± 0.90 b |
| Malvidin-3-glc | 89.68 ± 8.97 a | 101.81 ± 5.10 a | 99.17 ± 3.46 a | 82.84 ± 8.04 a | 80.27 ± 17.19 a | 89.45 ± 8.15 a |
| Total non-acylated | 133.42 ± 15.69 a | 153.68 ± 9.56 a | 148.77 ± 4.45 a | 107.53 ± 11.53 a | 115.40 ± 18.82 a | 123.29 ± 11.78 a |
| Delphinidin-3-acglc | 2.51 ± 0.24 a | 2.68 ± 0.13 a | 2.66 ± 0.07 a | 2.39 ± 0.19 a | 2.48 ± 0.38 a | 2.64 ± 0.16 a |
| Cyanidin-3-acglc | 1.35 ± 0.00 a | 1.37 ± 0.00 b | 1.36 ± 0.01 b | 1.36 ± 0.01 a | 1.37 ± 0.01 a | 1.38 ± 0.01 a |
| Petunidin-3-acglc | 2.61 ± 0.20 a | 2.67 ± 0.15 a | 2.66 ± 0.04 a | 2.59 ± 0.23 a | 2.64 ± 0.44 a | 2.77 ± 0.19 a |
| Peonidin-3-acglc | 2.12 ± 0.07 a | 2.60 ± 0.26 b | 2.41 ± 0.02 ab | 1.74 ± 0.10 a | 1.81 ± 0.17 a | 1.90 ± 0.09 a |
| Malvidin-3-acglc | 5.93 ± 0.46 a | 6.24 ± 0.09 a | 5.95 ± 0.19 a | 6.73 ± 0.44 a | 6.25 ± 0.94 a | 6.49 ± 0.30 a |
| Delphinidin-3-cmglc | 3.76 ± 0.35 a | 4.28 ± 0.37 a | 4.09 ± 0.13 a | 3.81 ± 0.57 a | 3.59 ± 0.68 a | 4.53 ± 0.58 a |
| Cyanidin-3-cmglc | 1.79 ± 0.09 a | 2.09 ± 0.17 b | 2.11 ± 0.09 b | 1.79 ± 0.11 a | 1.89 ± 0.29 a | 2.04 ± 0.15 a |
| Petunidin-3-cmglc | 2.90 ± 0.19 a | 3.30 ± 0.16 b | 3.11 ± 0.02 ab | 2.86 ± 0.35 a | 3.19 ± 0.45 a | 3.19 ± 0.38 a |
| Peonidin-3-cmglc | 2.37 ± 0.11 a | 2.91 ± 0.23 b | 2.82 ± 0.18 b | 2.28 ± 0.20 a | 2.44 ± 0.48 a | 2.66 ± 0.29 a |
| Malvidin-3-cis-cmglc | 1.71 ± 0.03 a | 1.74 ± 0.01 a | 1.70 ± 0.05 a | 1.82 ± 0.02 b | 1.70 ± 0.09 ab | 1.68 ± 0.06 a |
| Malvidin-3-trans-cmglc | 9.33 ± 0.46 a | 10.37 ± 0.38 b | 9.52 ± 0.33 a | 9.84 ± 1.52 a | 11.45 ± 2.60 a | 11.10 ± 2.30 a |
| Malvidin-3-cfglc | 1.99 ± 0.09 ab | 2.23 ± 0.17 b | 1.88 ± 0.17 a | 1.59 ± 0.06 a | 1.59 ± 0.26 a | 1.63 ± 0.08 a |
| Total acylated | 38.37 ± 2.22 a | 42.48 ± 0.97 b | 40.28 ± 0.45 ab | 38.80 ± 3.65 a | 40.41 ± 6.21 a | 42.01 ± 4.35 a |
| Total anthocyanins | 171.80 ± 17.75 a | 193.92 ± 14.13 a | 189.04 ± 4.81 a | 146.33 ± 15.18 a | 155.81 ± 24.83 a | 165.30 ± 16.01 a |
| Vitisin A | 2.00 ± 0.16 b | 1.73 ± 0.04 a | 1.68 ± 0.03 a | 1.51 ± 0.02 a | 1.53 ± 0.04 a | 1.55 ± 0.04 a |
| Vitisin B | 1.97 ± 0.12 a | 2.18 ± 0.18 a | 2.19 ± 0.05 a | 1.78 ± 0.05 a | 1.85 ± 0.23 a | 1.99 ± 0.08 a |
| 2019 | 2020 | |||||
|---|---|---|---|---|---|---|
| Control | MeJ | MeJ+Ur | Control | MeJ | MeJ+Ur | |
| Flavonols | ||||||
| Myricetin-3-glcU | 12.16 ± 1.20 a | 10.40 ± 1.63 a | 12.29 ± 1.28 a | 6.64 ± 0.39 a | 6.71 ± 0.62 a | 7.94 ± 1.24 a |
| Myricetin-3-gal | 15.56 ± 0.34 b | 13.33 ± 1.19 a | 13.81 ± 0.85 a | 8.14 ± 1.05 a | 9.49 ± 1.06 a | 13.33 ± 1.72 b |
| Myricetin-3-glc | 110.56 ± 6.68 a | 105.43 ± 17.27 a | 119.18 ± 6.25 a | 31.94 ± 6.38 a | 47.86 ± 5.78 a | 65.18 ± 11.97 b |
| Quercetin-3-glcU | 85.40 ± 11.76 b | 60.07 ± 6.79 a | 53.31 ± 9.82 a | 11.35 ± 1.11 a | 13.12 ± 1.76 a | 14.89 ± 2.64 a |
| Quercetin-3-glc | 94.97 ± 11.20 b | 74.64 ± 6.63 a | 75.28 ± 8.84 a | 57.77 ± 6.23 a | 76.74 ± 9.28 b | 83.88 ± 10.62 b |
| Laricitrin-3-glc | 17.50 ± 1.22 a | 15.95 ± 1.78 a | 17.03 ± 0.62 a | 10.79 ± 0.37 a | 11.79 ± 1.22 a | 15.16 ± 2.29 b |
| Kaempferol-3-gal | 1.58 ± 0.23 b | 1.30 ± 0.23 ab | 1.12 ± 0.19 a | 0.16 ± 0.01 a | 0.19 ± 0.03 a | 0.18 ± 0.02 a |
| Kaempferol-3-glcU+3-glc | 7.24 ± 1.14 b | 4.95 ± 0.61 a | 5.09 ± 0.88 a | 0.70 ± 0.10 a | 0.78 ± 0.07 a | 0.99 ± 0.10 b |
| Isorhamnetin-3-glc | 1.73 ± 0.24 a | 1.66 ± 0.28 a | 1.76 ± 0.13 a | 0.23 ± 0.04 a | 0.38 ± 0.04 b | 0.60 ± 0.06 c |
| Syringetin-3-glc | 11.25 ± 1.06 a | 10.67 ± 1.73 a | 11.46 ± 0.51 a | 8.92 ± 0.59 a | 10.40 ± 1.24 ab | 12.92 ± 2.90 b |
| Free-myricetin | 12.56 ± 0.46 a | 15.85 ± 2.44 a | 13.38 ± 1.24 a | 18.61 ± 3.15 a | 30.71 ± 5.01 b | 25.61 ± 3.85 ab |
| Free-quercetin | 18.85 ± 1.69 b | 18.73 ± 3.00 b | 11.57 ± 2.19 a | 14.36 ± 1.39 a | 17.09 ± 2.46 ab | 19.22 ± 2.32 b |
| Free-kaempferol | 10.09 ± 0.69 b | 11.42 ± 1.48 b | 7.56 ± 0.60 a | 3.95 ± 0.32 a | 3.93 ± 0.09 a | 4.70 ± 0.38 b |
| Free-laricitrin | 2.34 ± 0.06 a | 2.36 ± 0.22 a | 2.08 ± 0.11 a | 4.70 ± 0.29 a | 5.37 ± 1.12 a | 5.67 ± 0.51 a |
| Free-isorhamnetin+syringetin | 0.54 ± 0.05 ab | 0.64 ± 0.07 b | 0.48 ± 0.08 a | 0.38 ± 0.03 a | 0.40 ± 0.05 a | 0.47 ± 0.10 a |
| Total flavonols | 402.34 ± 29.87 a | 343.84 ± 40.47 a | 361.02 ± 38.48 a | 178.57 ± 6.30 a | 225.67 ± 55.20 ab | 277.97 ± 53.04 b |
| Flavanols | ||||||
| Catechin | 16.62 ± 1.12 a | 18.37 ± 2.85 a | 20.49 ± 3.04 a | 8.18 ± 1.57 a | 8.17 ± 1.05 a | 8.90 ± 1.07 a |
| Epicatechin | 19.02 ± 1.22 ab | 18.49 ± 3.53 a | 23.16 ± 0.58 b | 10.07 ± 1.46 a | 14.32 ± 2.04 b | 11.76 ± 1.44 ab |
| Epicatechin-3-gallate | 17.24 ± 1.84 a | 16.71 ± 3.22 a | 18.78 ± 3.05 a | n.d. | n.d. | n.d. |
| Epigallocatechin | 1.50 ± 0.23 a | 2.32 ± 0.37 b | 1.98 ± 0.32 ab | 6.14 ± 0.93 a | 7.45 ± 0.73 a | 10.44 ± 1.82 b |
| Procyanidin B1 | 7.47 ± 0.96 a | 15.93 ± 1.11 c | 12.23 ± 1.36 b | 2.64 ± 0.42 a | 4.46 ± 0.57 b | 5.03 ± 1.00 b |
| Procyanidin B2 | 16.34 ± 1.50 b | 8.06 ± 1.53 a | 24.58 ± 3.75 c | n.d. | n.d. | n.d. |
| Total flavanols | 81.99 ± 2.40 a | 87.77 ± 16.59 a | 96.98 ± 9.19 a | 26.13 ± 4.77 a | 35.72 ± 3.47 ab | 36.91 ± 6.16 b |
| Hydroxybenzoic acid | ||||||
| Gallic acid | 29.84 ± 4.11 b | 20.17 ± 2.87 a | 29.69 ± 5.74 b | 14.46 ± 1.04 a | 18.89 ± 1.26 a | 18.10 ± 3.73 a |
| Hydroxycinnamic acids (HCAs) | ||||||
| trans-Caftaric acid | 4.42 ± 0.53 b | 2.27 ± 0.51 a | 7.48 ± 0.46 c | 9.19 ± 1.00 a | 12.23 ± 1.04 b | 9.88 ± 1.52 ab |
| trans+cis-Coutaric acids | 2.65 ± 0.29 b | 1.70 ± 0.32 a | 4.70 ± 0.22 c | 7.07 ± 0.71 a | 8.98 ± 0.83 a | 7.04 ± 1.36 a |
| trans-Fertaric acid | 1.12 ± 0.10 a | 0.93 ± 0.14 a | 1.07 ± 0.09 a | 1.48 ± 0.04 a | 1.90 ± 0.28 b | 2.06 ± 0.21 b |
| Caffeic acid | 30.43 ± 0.71 b | 22.49 ± 2.48 a | 30.02 ± 0.61 b | 12.11 ± 2.28 a | 14.50 ± 3.05 a | 15.42 ± 3.01 a |
| p-Coumaric acid | 10.52 ± 0.98 ab | 7.95 ± 0.10 a | 12.38 ± 2.07 b | 7.30 ± 1.46 a | 8.35 ± 1.55 ab | 11.45 ± 2.08 b |
| Ferulic acid | 2.31 ± 0.29 b | 1.83 ± 0.31 ab | 1.71 ± 0.16 a | 2.08 ± 0.37 a | 2.63 ± 0.30 ab | 3.14 ± 0.40 b |
| Total HCAs | 52.19 ± 3.53 a | 43.97 ± 10.35 a | 54.01 ± 7.90 a | 39.24 ± 2.48 a | 48.36 ± 3.65 ab | 50.09 ± 6.85 b |
| Stilbenes | ||||||
| trans-Piceid | 3.55 ± 0.22 a | 3.43 ± 0.56 a | 3.83 ± 0.20 a | 0.87 ± 0.08 a | 1.56 ± 0.20 b | 2.10 ± 0.41 c |
| cis-Piceid | 0.24 ± 0.04 a | 0.47 ± 0.06 b | 0.41 ± 0.03 b | 0.95 ± 0.13 a | 0.87 ± 0.09 a | 1.35 ± 0.16 b |
| trans-Resveratrol | 0.58 ± 0.02 a | 0.74 ± 0.12 b | 0.47 ± 0.02 a | 1.87 ± 0.07 a | 2.96 ± 0.22 b | 3.16 ± 0.38 b |
| cis-Resveratrol | 0.63 ± 0.10 a | 0.67 ± 0.06 a | 0.83 ± 0.02 b | 0.50 ± 0.04 a | 0.73 ± 0.15 ab | 0.77 ± 0.16 b |
| Total stilbenes | 5.15 ± 0.43 a | 5.23 ± 1.11 a | 5.67 ± 0.37 a | 4.28 ± 0.37 a | 5.93 ± 0.91 ab | 7.65 ± 1.43 b |
| 2019 | 2020 | |||||
|---|---|---|---|---|---|---|
| Control | MeJ | MeJ+Ur | Control | MeJ | MeJ+Ur | |
| Aspartic acid | 0.07 ± 0.02 a | 1.40 ± 0.46 b | 4.80 ± 0.15 c | 7.27 ± 0.65 b | 6.67 ± 0.56 b | 4.63 ± 1.29 a |
| Glutamic acid | 3.44 ± 0.88 a | 5.73 ± 2.25 a | 25.45 ± 4.96 b | 16.38 ± 1.32 b | 17.88 ± 4.51 b | 10.46 ± 1.74 a |
| Asparagine | 3.36 ± 0.82 a | 5.78 ± 1.23 b | 15.01 ± 0.09 c | 8.22 ± 1.31 a | 7.62 ± 1.12 a | 6.79 ± 1.50 a |
| Serine | 3.17 ± 1.01 a | 3.13 ± 1.49 a | 6.72 ± 0.06 b | 7.71 ± 1.11 a | 7.44 ± 1.00 a | 5.96 ± 0.66 a |
| Glutamine | 2.79 ± 0.20 a | 1.99 ± 0.95 a | 16.05 ± 2.28 b | 6.89 ± 1.01 a | 5.27 ± 1.33 a | 6.85 ± 1.77 a |
| Histidine | 5.25 ± 1.10 a | 5.51 ± 1.44 a | 12.75 ± 2.04 b | 13.10 ± 2.38 b | 7.83 ± 1.43 a | 10.88 ± 1.57 ab |
| Glycine | 6.48 ± 0.41 a | 8.85 ± 1.89 a | 19.96 ± 1.52 b | 15.29 ± 2.04 a | 14.80 ± 3.26 a | 12.48 ± 1.18 a |
| Threonine+Citrulline | 1.82 ± 0.22 a | 3.46 ± 1.13 a | 23.84 ± 4.80 b | 10.62 ± 1.23 a | 8.12 ± 1.82 a | 8.61 ± 0.88 a |
| Arginine | 6.02 ± 0.28 a | 6.51 ± 0.36 a | 5.65 ± 0.86 a | 7.09 ± 1.85 b | 4.34 ± 0.69 a | 8.09 ± 0.87 b |
| Alanine | 3.52 ± 0.99 a | 8.05 ± 3.37 a | 23.08 ± 5.78 b | 26.21 ± 5.20 a | 21.56 ± 2.53 a | 18.76 ± 2.64 a |
| γ-Aminobutyric acid | 9.06 ± 1.37 a | 16.69 ± 5.17 a | 117.24 ± 9.92 b | 14.24 ± 1.83 b | 15.17 ± 2.61 b | 6.29 ± 0.17 a |
| Proline | 647.05 ± 45.92 a | 726.77 ± 110.61 a | 985.59 ± 96.06 b | 2172.04 ± 120.58 ab | 1816.80 ± 218.65 a | 2397.73 ± 272.59 b |
| Tyrosine | 0.63 ± 0.05 a | 1.96 ± 1.72 a | 4.59 ± 1.18 b | 6.68 ± 0.67 c | 4.94 ± 0.80 b | 3.19 ± 0.15 a |
| Valine | 0.67 ± 0.06 a | 2.32 ± 1.72 ab | 3.02 ± 0.24 b | 7.46 ± 0.96 b | 6.34 ± 0.87 b | 4.22 ± 0.45 a |
| Methionine | 0.56 ± 0.10 a | 0.86 ± 0.47 a | 1.53 ± 0.26 b | 1.69 ± 0.28 b | 1.39 ± 0.28 b | 0.58 ± 0.11 a |
| Cysteine | 0.44 ± 0.06 b | 0.27 ± 0.06 a | 0.31 ± 0.09 ab | 0.36 ± 0.04 a | 0.38 ± 0.06 a | 0.39 ± 0.09 a |
| Isoleucine+Tryptophan | 0.93 ± 0.07 a | 1.87 ± 0.96 a | 4.72 ± 0.27 b | 7.60 ± 0.76 b | 7.25 ± 0.90 b | 4.59 ± 0.50 a |
| Leucine | 1.40 ± 0.32 a | 4.84 ± 1.00 b | 4.86 ± 0.56 b | 12.94 ± 2.44 b | 7.72 ± 0.90 a | 5.14 ± 0.44 a |
| Phenylalanine | 0.94 ± 0.19 a | 2.77 ± 0.68 b | 3.29 ± 0.26 b | 9.52 ± 1.49 b | 5.66 ± 0.72 a | 4.58 ± 0.39 a |
| Ornithine | 3.26 ± 0.25 a | 49.27 ± 23.18 b | 54.72 ± 3.28 b | 33.74 ± 3.30 b | 16.98 ± 1.40 a | 21.96 ± 3.37 a |
| Lysine | 2.42 ± 0.40 a | 8.03 ± 0.87 b | 7.39 ± 0.36 b | 26.88 ± 3.35 b | 19.89 ± 3.20 a | 15.34 ± 0.40 a |
| Total AA | 703.27 ± 41.79 a | 866.04 ± 121.48 a | 1340.55 ± 106.41 b | 2411.95 ± 135.64 ab | 2004.05 ± 237.31 a | 2557.51 ± 285.93 b |
| Total AA without Pro | 56.22 ± 7.14 a | 139.27 ± 27.70 b | 357.97 ± 19.64 b | 239.91 ± 23.20 b | 187.25 ± 21.38 a | 159.78 ± 13.78 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Lázaro, M.; Sáenz de Urturi, I.; Murillo-Peña, R.; Marín-San Román, S.; Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Effect of Methyl Jasmonate and Methyl Jasmonate Plus Urea Foliar Applications on Wine Phenolic, Aromatic and Nitrogen Composition. Beverages 2022, 8, 52. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages8030052
González-Lázaro M, Sáenz de Urturi I, Murillo-Peña R, Marín-San Román S, Pérez-Álvarez EP, Rubio-Bretón P, Garde-Cerdán T. Effect of Methyl Jasmonate and Methyl Jasmonate Plus Urea Foliar Applications on Wine Phenolic, Aromatic and Nitrogen Composition. Beverages. 2022; 8(3):52. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages8030052
Chicago/Turabian StyleGonzález-Lázaro, Miriam, Itziar Sáenz de Urturi, Rebeca Murillo-Peña, Sandra Marín-San Román, Eva P. Pérez-Álvarez, Pilar Rubio-Bretón, and Teresa Garde-Cerdán. 2022. "Effect of Methyl Jasmonate and Methyl Jasmonate Plus Urea Foliar Applications on Wine Phenolic, Aromatic and Nitrogen Composition" Beverages 8, no. 3: 52. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages8030052