Stoichiometric Ratio Controlled Dimension Transition and Supramolecular Chirality Enhancement in a Two-Component Assembly System
Abstract
:1. Introduction
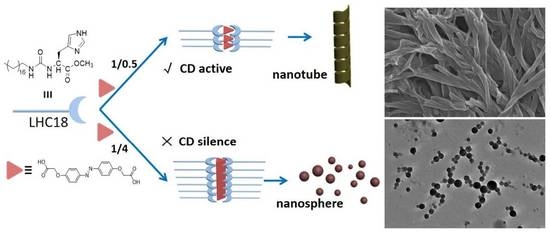
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.H.; Yang, Y.S.; Yang, Y.; Kim, D.S.; Yuan, A.; Tian, X.; Ophus, C.; Sun, F.; Schmid, A.K.; Nathanson, M.; et al. Observing crystal nucleation in four dimensions using atomic electron tomography. Nature 2019, 570, 500–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, J.S.B.; Smalyukh, I.I. Three-dimensional crystals of adaptive knots. Science 2019, 365, 1449–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katan, C.; Mercier, N.; Even, J. Quantum and dielectric confinement effects in lower-dimensional hybrid perovskite semiconductors. Chem. Rev. 2019, 119, 3140–3192. [Google Scholar] [CrossRef]
- Huo, D.; Kim, M.J.; Lyu, Z.H.; Shi, Y.F.; Wiley, B.J.; Xia, Y.N. One dimensional metal nanostructures: From colloidal syntheses to applications. Chem. Rev. 2019, 119, 8972–9073. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Su, B.; Jiang, L. Recent advances in one-dimensional assembly of nanoparticles. Chem. Soc. Rev. 2019, 48, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Waterhouse, G.I.N.; Chen, G.B.; Xiong, X.Y.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Two-dimensional-related catalytic materials for solar-driven conversion of cox into valuable chemical feedstocks. Chem. Soc. Rev. 2019, 48, 1972–2010. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Li, M.J.; Li, C.X. Intensifying solar-thermal harvest of low-dimension biologic nanostructures for electric power and solar desalination. Nano Energy 2018, 50, 308–315. [Google Scholar] [CrossRef]
- Brouhard, G.J.; Rice, L.M. Microtubule dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463. [Google Scholar] [CrossRef]
- Amabilino, D.B.; Smith, D.K.; Steed, J.W. Supramolecular materials. Chem. Soc. Rev. 2017, 46, 2404–2420. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular helical systems: Helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Onogi, S.; Shigemitsu, H.; Hamachi, I. Chemically reactive supramolecular hydrogel coupled with a signal amplification system for enhanced analyte sensitivity. J. Am. Chem. Soc. 2015, 137, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.F.; Wang, T.Y.; Liu, M.H. Tuning soft nanostructures in self-assembled supramolecular gels: From morphology control to morphology-dependent functions. Small 2015, 11, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Zhang, L.; Wang, T.Y. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Hao, A.Y.; Xing, P.Y. X⋅⋅⋅X halogen bond-induced supramolecular helices. Angew. Chem. Int. Ed. 2021, 61, e202113786. [Google Scholar]
- Tsai, M.F.; Chang, S.H.G.; Cheng, F.Y.; Shanmugam, V.; Cheng, Y.S.; Su, C.H.; Yeh, C.S. Au nanorod design as light-absorber in the first and second biological near-infrared windows for in vivo photothermal therapy. ACS Nano 2013, 7, 5330–5342. [Google Scholar] [CrossRef]
- Liu, G.F.; Sheng, J.H.; Teo, W.L.; Yang, G.B.; Wu, H.W.; Li, Y.X.; Zhao, Y.L. Control on dimensions and supramolecular chirality of self-assemblies through light and metal Ions. J. Am. Chem. Soc. 2018, 140, 16275–16283. [Google Scholar] [CrossRef]
- Kumar, J.; Nakashima, T.; Tsumatori, H.; Kawai, T. Circularly polarized luminescence in chiral aggregates: Dependence of morphology on luminescence dissymmetry. J. Phys. Chem. Lett. 2014, 5, 316–321. [Google Scholar] [CrossRef]
- Zhou, P.; Shi, R.F.; Yao, J.F.; Sheng, C.F.; Li, H. Supramolecular self-assembly of nucleotide–metal coordination complexes: From simple molecules to nanomaterials. Coord. Chem. Rev. 2015, 292, 107–143. [Google Scholar] [CrossRef]
- Xing, P.Y.; Zhao, Y.L. Controlling supramolecular chirality in multicomponent self-assembled systems. Acc. Chem. Res. 2018, 51, 2324–2334. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Chen, C.F.; Wang, T.Y.; Liu, M.H. Supramolecular chirality of the two-component supramolecular copolymer gels: Who determines the handedness? Langmuir 2016, 32, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.J.; Wei, Z.X. Supramolecular helices: Chirality transfer from conjugated molecules to structures. Adv. Mater. 2013, 25, 6039–6049. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yu, H.F.; Li, Q. Supramolecular chirality transfer toward chiral aggregation: Asymmetric hierarchical self-assembly. Adv. Sci. 2021, 8, 2002132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.E.; Li, Y.Z.; Hao, A.Y.; Xing, P.Y. Multi-modal chiral superstructures in self-assembled anthracene-terminal amino acids with predictable and adjustable chiroptical activities and color evolution. Angew. Chem. Int. Ed. 2021, 60, 3138–3147. [Google Scholar] [CrossRef]
- Wu, A.L.; Guo, Y.X.; Li, X.B.; Xue, H.M.; Fei, J.B.; Li, J.B. Co-assembled supramolecular gel of dipeptide and pyridine derivatives with controlled chirality. Angew. Chem. Int. Ed. 2021, 60, 2099–2103. [Google Scholar] [CrossRef] [PubMed]
- Adawy, A. Functional chirality: From small molecules to supramolecular assemblies. Symmetry 2022, 14, 292. [Google Scholar] [CrossRef]
- Colquhoun, C.; Draper, E.R.; Eden, E.G.B.; Cattoz, B.N.; Morris, K.L.; Chen, L.; McDonald, T.O.; Terry, A.E.; Griffiths, P.C.; Serpell, L.C.; et al. The effect of self-sorting and Co-assembly on the mechanical properties of low molecular weight hydrogels. Nanoscale 2014, 6, 13719–13725. [Google Scholar] [CrossRef] [Green Version]
- Tena-Solsona, M.; Alonso-de Castro, S.; Miravet, J.F.; Escuder, B. Co-assembly of tetrapeptides into complex pH-responsive molecular hydrogel networks. J. Mater. Chem. B 2014, 2, 6192–6197. [Google Scholar] [CrossRef]
- Das, P.; Yuran, S.; Yan, J.; Lee, P.S.; Reches, M. Sticky tubes and magnetic hydrogels co-assembled by a short peptide and melanin- like nanoparticles. Chem. Commun. 2015, 51, 5432–5435. [Google Scholar] [CrossRef]
- Matsumoto, K.; Shundo, A.; Ohno, M.; Saruhashi, K.; Miyachi, N.; Tsuruzoe, N.; Tanaka, K. Sol-gel transition accelerated by the co-assembly of two components in supramolecular hydrogels. Phys. Chem. Chem. Phys. 2015, 17, 26724–26730. [Google Scholar] [CrossRef]
- Khatua, D.; Maiti, R.; Dey, J. A supramolecular hydrogel that responds to biologically relevant stimuli. Chem. Commun. 2006, 47, 4903–4905. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.J.; Chen, G.S.; Liu, X.X.; Chen, W.X.; Chen, F.; Jiang, M. Photoresponsive pseudopolyrotaxane hydrogels based on competition of host−guest interactions. Angew. Chem. Int. Ed. 2010, 49, 4409–4413. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Chen, W.J.; Zhu, X.F.; Song, X.; Liu, M.H. Self-assembly and circularly polarized luminescence from achiral pyrene-adamantane conjugates by selective inclusion with cyclodextrins. J. Phys. Chem. Lett. 2021, 12, 7491–7496. [Google Scholar] [CrossRef] [PubMed]
- Palmans, A.R.A.; Meijer, E.W. Amplification of chirality in dynamic supramolecular aggregates. Angew. Chem. Int. Ed. 2007, 46, 8948–8968. [Google Scholar] [CrossRef]
- Dijken, D.J.; Beierle, J.M.; Stuart, M.C.A.; Szymański, W.; Browne, W.R.; Feringa, B.L. Autoamplification of molecular chirality through the induction of supramolecular chirality. Angew. Chem. Int. Ed. 2014, 53, 5073–5077. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Zhu, L.Y.; Ji, W.; Feng, C.L.; Wei, Z.X. Inversion of the supramolecular chirality of nanofibrous structures through Co-assembly with achiral molecules. Angew. Chem. Int. Ed. 2016, 55, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Raciti, D.; Randazzo, R.; Gangemi, C.M.A.; Raudino, A.; D’Urso, A.; Fragalà, M.E.; Purrello, R. Chirality enhancement of porphyrin supramolecular assembly driven by a template preorganization effect. Angew. Chem. Int. Ed. 2018, 57, 10656–10660. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.S.; Chen, K.; Zhang, H.Q.; Liu, Y. Nano-supramolecular assemblies constructed from water-soluble bis(calix[5]arenes) with porphyrins and their photoinduced electron transfer properties. Chem. Asian J. 2009, 4, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Sortino, G.; Randazzo, R.; Pisagatti, I.; Notti, A.; Fragalà, M.E.; Parisi, M.F.; D’Urso, A.; Purrello, R. Long-range chiral induction by a fully noncovalent approach in supramolecular porphyrin–calixarene assemblies. Chem. Eur. J. 2020, 26, 3515–3518. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Timoneda, M.A.; Crego-Calama, M.; Reinhoudt, D.N. Supramolecular chirality of self-assembled systems in solution. Chem. Soc. Rev. 2004, 33, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Notti, A.; Pappalardo, S.; Parisi, M.; Pisagatti, I. Recognition in water of bioactive substrates by a sulphonato p-tert-butylcalix[5]arene. Supramol. Chem. 2014, 26, 597–600. [Google Scholar] [CrossRef]
- Borovkov, V.V.; Lintuluoto, J.M.; Sugiura, M.; Inoue, Y.; Kuroda, R. Remarkable stability and enhanced optical activity of a chiral supramolecular bis-porphyrin tweezer in both solution and solid state. J. Am. Chem. Soc. 2002, 124, 11282–11283. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, A.; Marino, N.; Gaeta, M.; Rizzo, M.S.; Cristaldi, D.A.; Fragalà, M.E.; Pappalardo, S.; Gattuso, G.; Notti, A.; Parisi, M.F.; et al. Porphyrin stacks as an efficient molecular glue to induce chirality in hetero-component calixarene–porphyrin assemblies. New J. Chem. 2017, 41, 8078–8083. [Google Scholar] [CrossRef]
- Ji, L.K.; Sang, Y.T.; Ouyang, G.H.; Yang, D.; Duan, P.F.; Jiang, Y.Q.; Liu, M.H. Cooperative chirality and sequential energy transfer in a supramolecular light-harvesting nanotube. Angew. Chem. Int. Ed. 2019, 58, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Kameta, N.; Ishikawa, K.; Masuda, M.; Asakawa, M.; Shimizu, T. Soft nanotubes acting as a light-harvesting antenna system. Chem. Mater. 2012, 24, 209–214. [Google Scholar] [CrossRef]
- Cao, Z.Z.; Hao, A.Y.; Xing, P.Y. Photoresponsive chiral vesicles as a light harvesting matrix with tunable chiroptical properties. Nanoscale 2021, 13, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Duan, P.F.; Zhang, L.; Liu, M.H. Chirality and energy transfer amplified circularly polarized luminescence in composite nanohelix. Nat. Commun. 2017, 8, 15727. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.H.; Han, J.L.; Duan, P.F.; Liu, M.H. New perspectives to trigger and modulate circularly polarized luminescence of complex and aggregated systems: Energy transfer, photon upconversion, charge transfer, and organic radical. Acc. Chem. Res. 2020, 53, 1279–1292. [Google Scholar] [CrossRef]
- Cao, Z.Z.; Wang, B.; Zhu, F.; Hao, A.Y.; Xing, P.Y. Solvent-processed circularly polarized luminescence in light-harvesting coassemblies. ACS Appl. Mater. Interfaces 2020, 12, 34470–34478. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.; Fang, L.; Shi, L.; Tang, Z.Y.; Lu, H.Y.; Chen, C.F. Chiral nanoparticles with full-color and white CPL properties based on optically stable helical aromatic imide enantiomers. ACS Appl. Mater. Interfaces 2018, 10, 8225–8230. [Google Scholar] [CrossRef]
- Nishikawa, T.; Nagata, Y.; Suginome, M. Poly(quinoxaline-2,3-diyl) as a multifunctional chiral scaffold for circularly polarized luminescent materials: Color tuning, energy transfer, and switching of the CPL handedness. ACS Macro Lett. 2017, 6, 431–435. [Google Scholar] [CrossRef]
- Niu, D.; Ji, L.K.; Ouyang, G.H.; Liu, M.H. Histidine proton shuttle-initiated switchable inversion of circularly polarized luminescence. ACS Appl. Mater. Interfaces 2020, 12, 18148–18156. [Google Scholar] [CrossRef] [PubMed]
- Amako, T.; Nakabayashi, K.; Mori, T.; Inoue, Y.; Fujiki, M.; Imai, Y. Sign inversion of circularly polarized luminescence by geometry manipulation of four naphthalene units introduced into a tartaric acid scaffold. Chem. Commun. 2014, 50, 12836–128399. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.C.; Wang, T.Y.; Liu, M.H. H-bond and π–π stacking directed self-assembly of two-component supramolecular nanotubes: Tuning length, diameter and wall thickness. Chem. Commun. 2014, 50, 2096–2099. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.H.; Wu, Z.; Li, P.Y.; Niu, D.; Liu, M.H.; Yin, M.Z. Triple-modulated chiral inversion of Co-assembly system based on alanine amphiphile and cyanostilbene derivative. ACS Appl. Mater. Interfaces 2021, 13, 18047–18055. [Google Scholar] [CrossRef]
- Chen, J.; Wang, T.Y.; Liu, M.H. Chaperone gelator for the chiral self-assembly of all proteinogenic amino acids and their enantiomers. Chem. Commun. 2016, 52, 6123–6126. [Google Scholar] [CrossRef]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef]
- Cao, H.; Jiang, J.; Zhu, X.F.; Duan, P.F.; Liu, M.H. Hierarchical Co-assembly of chiral lipid nanotubes with an azobenzene derivative: Optical and chiroptical switching. Soft Matter 2011, 7, 4654. [Google Scholar] [CrossRef]
- Duan, P.F.; Li, Y.G.; Li, L.C.; Deng, J.G.; Liu, M.H. Multiresponsive chiroptical switch of an azobenzene-containing lipid: Solvent, temperature, and photoregulated supramolecular chirality. J. Phys. Chem. B 2011, 115, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.K.; Ouyang, G.H.; Liu, M.H. Binary supramolecular gel of achiral azobenzene with a chaperone gelator: Chirality transfer, tuned morphology, and chiroptical property. Langmuir 2017, 33, 12419–12426. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.P.; Wee, T.S.A.; Chin, W.S. Three-dimensional self-assembled monolayer (3D SAM) of n-alkanethiols on copper nanoclusters. J. Phys. Chem. B 2004, 108, 11001–11010. [Google Scholar] [CrossRef]
- Shi, H.F.; Ying, Z.; Zhang, X.Q.; Yong, Z.; Xu, Y.Z.; Zhou, S.R.; Wang, D.J.; Han, C.C.; Xu, D.F. Packing mode and conformational transition of alkyl side chains in N-alkylated poly(p-benzamide) comb-like polymer. Polymer 2014, 45, 6299–6307. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Verma, A.L.; Yoneyama, M.; Nakashima, K.; Iriyama, K.; Ozaki, Y. Molecular orientation and aggregation in langmuir-blodgett films of 5-(4-N-Octadecylpyridyl)-10,15,20-tri-p-tolylporphyrin studied by ultraviolet-visible and infrared spectroscopies. Langmuir 1997, 13, 4422–4427. [Google Scholar] [CrossRef]
- Li, H.; Duan, Z.; Yang, Y.; Xu, F.; Chen, M.; Liang, T.; Bai, Y.; Li, R. Regulable Aggregation-Induced Emission Supramolecular Polymer and Gel Based on Self-sorting Assembly. Macromolecules 2020, 53, 4255–4263. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Liu, Y.; Fang, X.; Ma, L.; Wang, Y.; Ji, L. Stoichiometric Ratio Controlled Dimension Transition and Supramolecular Chirality Enhancement in a Two-Component Assembly System. Gels 2022, 8, 269. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050269
Zhang P, Liu Y, Fang X, Ma L, Wang Y, Ji L. Stoichiometric Ratio Controlled Dimension Transition and Supramolecular Chirality Enhancement in a Two-Component Assembly System. Gels. 2022; 8(5):269. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050269
Chicago/Turabian StyleZhang, Penghui, Yiran Liu, Xinkuo Fang, Li Ma, Yuanyuan Wang, and Lukang Ji. 2022. "Stoichiometric Ratio Controlled Dimension Transition and Supramolecular Chirality Enhancement in a Two-Component Assembly System" Gels 8, no. 5: 269. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050269