Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications
Abstract
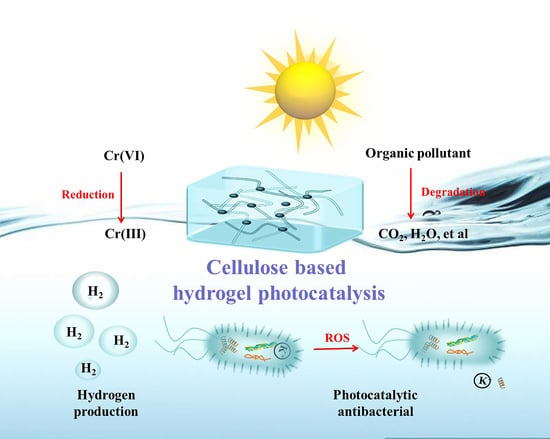
:1. Introduction
2. Characteristics of Cellulose-Based Hydrogel Photocatalytic Composites
2.1. High Adsorption
2.2. Dispersibility
2.3. Morphological Adjuvants
3. Preparation of Cellulose-Based Hydrogel Photocatalytic Composites
3.1. Physical Cross-Linking Method
3.2. Chemical Cross-Linking Method
3.3. Radiation Cross-Linking Method
4. Classification of Cellulose-Based Hydrogel Photocatalytic Materials
4.1. Metal Oxide Semiconductor Composites
4.2. Metal Sulfide (Chloride) Semiconductor Composites
4.3. Organic Semiconductor Composites
5. Application of Cellulose-Based Hydrogel Photocatalytic Materials
5.1. Wastewater Treatment
5.1.1. Removal of Dyes and Heavy Metal Ions
5.1.2. Degradation of Antibiotics
5.1.3. Antibacterial Properties
5.2. Energy
5.2.1. Hydrogen Energy
5.2.2. Food Packaging
6. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yakamercan, E.; Ari, A.; Aygün, A. Land application of municipal sewage sludge: Human health risk assessment of heavy metals. J. Clean. Prod. 2021, 319, 128568. [Google Scholar] [CrossRef]
- Su, H.C.; Liu, Y.S.; Pan, C.G.; Chen, J.; He, L.Y.; Ying, G.G. Persistence of antibiotic resistance genes and bacterial community changes in drinking water treatment system: From drinking water source to tap water. Sci. Total Environ. 2018, 616, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, Z.; Yuan, B.; Li, X.; Li, S.; Nguyen, T.T.; Guo, M.; Guo, Z. Fluorescent carbon dots crosslinked cellulose nanofibril/chitosan interpenetrating hydrogel system for sensitive detection and efficient adsorption of Cu (II) and Cr (VI). Chem. Eng. J. 2022, 430, 133154. [Google Scholar] [CrossRef]
- Alhalili, Z.; Romdhani, C.; Chemingui, H.; Smiri, M. Removal of dithioterethiol (DTT) from water by membranes of cellulose acetate (AC) and AC doped ZnO and TiO2 nanoparticles. J. Saudi Chem. Soc. 2021, 25, 101282. [Google Scholar] [CrossRef]
- Cui, J.; Li, J.F.; Cui, J.; Wang, W.; Wu, Y.; Xu, B.; Chang, Y.J.; Liu, X.J.; Li, H.; Yao, D.R. Removal effects of a biomass bottom ash composite on tailwater phosphate and its application in a rural sewage treatment plant. Sci. Total Environ. 2021, 812, 152549. [Google Scholar] [CrossRef]
- Calero-Cáceres, M.; Muniesa, M. Persistence of naturally occurring antibiotic resistance genes in the bacteria and bacteriophage fractions of wastewater. Water Res. 2016, 95, 11–18. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Khan, M.E. State-of-the-art developments in carbon-based metal nanocomposites as a catalyst: Photocatalysis. Nanoscale Adv. 2021, 3, 1887–1900. [Google Scholar] [CrossRef]
- He, X.; Antonelli, D. Recent Advances in Synthesis and Applications of Transition Metal Containing Mesoporous Molecular Sieves. Angew. Chem. Int. Ed. 2002, 41, 214–229. [Google Scholar] [CrossRef]
- Chaubey, S.; Singh, P.; Singh, C.; Shambhavi; Sharma, K.; Yadav, R.K.; Kumar, A.; Baeg, J.; Dwivedi, D.K.; Singh, A.P. Self-assembled protein/carbon nitride/sulfur hydrogel photocatalyst for highly selective solar chemical production. Mater. Lett. 2020, 259, 126752. [Google Scholar] [CrossRef]
- Koo, H.J.; Velev, O.D. Biomimetic photocatalytic reactor with a hydrogel-embedded microfluidic network. J. Mater. Chem. A 2013, 1, 11106–11110. [Google Scholar] [CrossRef]
- Bao, Z.T.; Xian, C.H.; Yuan, Q.J.; Liu, G.T.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.W.; Wang, H.Y.; Zhou, Q.Q.; Wu, M.M.; Zhang, M.; Li, C.; Shi, G.Q. Ultrahigh-Conductivity Polymer Hydrogels with Arbitrary Structures. Adv. Mater. 2017, 29, 1700974. [Google Scholar] [CrossRef]
- Job, N.; Théry, A.; Pirard, R.; Marien, J.; Kocon, L.; Rouzaud, J.-N.; Béguin, F.; Pirard, J.-P. Carbon aerogels, cryogels and xerogels: Influence of the drying method on the textural properties of porous carbon materials. Carbon 2005, 43, 2481–2494. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef] [Green Version]
- Dragan, E.S.; Dinu, M.V. Progress in Polysaccharide/Zeolites and Polysaccharide Hydrogel Composite Sorbents and Their Applications in Removal of Heavy Metal Ions and Dyes. Curr. Green Chem. 2015, 2, 342–353. [Google Scholar] [CrossRef]
- Hemmati, F.; Jafari, S.M.; Taheri, R.A. Optimization of homogenization-sonication technique for the production of cellulose nanocrystals from cotton linter. Int. J. Biol. Macromol. 2019, 137, 374–381. [Google Scholar] [CrossRef]
- Marin, D.C.; Vecchio, A.; Ludueña, L.N.; Fasce, D.; Alvarez, V.A.; Stefani, P.M. Revalorization of Rice Husk Waste as a Source of Cellulose and Silica. Fibers Polym. 2015, 16, 285–293. [Google Scholar] [CrossRef]
- Gullo, M.; La China, S.; Falcone, P.M.; Giudici, P. Biotechnological production of cellulose by acetic acid bacteria: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 6885–6898. [Google Scholar] [CrossRef]
- Rincon-Iglesias, M.; Lizundia, E.; Lanceros-Méndez, S. Water-Soluble Cellulose Derivatives as Suitable Matrices for Multifunctional Materials. Biomacromolecules 2019, 20, 2786–2795. [Google Scholar] [CrossRef] [PubMed]
- Kareem, S.A.; Dere, I.; Gungula, D.T.; Andrew, F.P.; Saddiq, A.M.; Adebayo, E.F.; Tame, V.T.; Kefas, H.M.; Joseph, J.; Patrick, D.O. Synthesis and Characterization of Slow-Release Fertilizer Hydrogel Based on Hydroxy Propyl Methyl Cellulose, Polyvinyl Alcohol, Glycerol and Blended Paper. Gels 2021, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.D.; Li, J.; Su, Y.; Li, Q.; Gao, B.Y.; Yue, Q.Y.; Zhou, W.Z. Adsorption and recycling of Cd(II) from wastewater using straw cellulose hydrogel beads. J. Ind. Eng. Chem. 2019, 80, 361–369. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Li, Z.X.; Li, W.Z.; Bian, F.G. Mesoporous cellulose/TiO2/SiO2/TiN-based nanocomposite hydrogels for efficient solar steam evaporation: Low thermal conductivity and high light-heat conversion. Cellulose 2020, 27, 481–491. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y. Removal of heavy metal ion by floatable hydrogel and reusability of its waste material in photocatalytic degradation of organic dyes. J. Environ. Chem. Eng. 2021, 9, 105316. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, J.G. Hierarchical-Structured Anatase-Titania/Cellulose Composite Sheet with High Photocatalytic Performance and Antibacterial Activity. Chem. A Eur. J. 2015, 21, 2568–2575. [Google Scholar] [CrossRef]
- Dhanya, G.; Palanisamy Uma, M.; Khadar Mohamed Meera Sherifffa, B.; Gangasalam, A. Biomass-Derived Dialdehyde Cellulose Cross-linked Chitosan-Based Nanocomposite Hydrogel with Phytosynthesized Zinc Oxide Nanoparticles for Enhanced Curcumin Delivery and Bioactivity. J. Agric. Food Chem. 2019, 67, 10880–10890. [Google Scholar]
- Azarniya, A.; Tamjid, E.; Eslahi, N.; Simchi, A. Modification of bacterial cellulose/keratin nanofibrous mats by a tragacanth gum-conjugated hydrogel for wound healing. Int. J. Biol. Macromol. 2019, 134, 280–289. [Google Scholar] [CrossRef]
- Dorishetty, P.; Balu, R.; Athukoralalage, S.S.; Greaves, T.L.; Mata, J.; De Campo, L.; Saha, N.; Zannettino, A.C.W.; Dutta, N.K.; Choudhury, N.R. Tunable biomimetic hydrogels from silk fibroin and nanocellulose. ACS Sustain. Chem. Eng. 2020, 8, 2375–2389. [Google Scholar] [CrossRef]
- Enawgaw, H.; Tesfaye, T.; Yilma, K.T.; Limeneh, D.Y. Synthesis of a Cellulose-Co-AMPS Hydrogel for Personal Hygiene Applications Using Cellulose Extracted from Corncobs. Gels 2021, 7, 236. [Google Scholar] [CrossRef]
- Arias, S.L.; Cheng, M.K.; Civantos, A.; Devorkin, J.; Jaramillo, C.; Allain, J.P. Ion-Induced Nanopatterning of Bacterial Cellulose Hydrogels for Biosensing and Anti-Biofouling Interfaces. ACS Appl. Nano Mater. 2020, 3, 6719–6728. [Google Scholar] [CrossRef]
- Yu, J.; Wang, A.C.; Zhang, M.; Lin, Z. Water treatment via non-membrane inorganic nanoparticles/cellulose composites. Mater. Today 2021, 50, 329–357. [Google Scholar] [CrossRef]
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Functionalization and Antibacterial Applications of Cellulose-Based Composite Hydrogels. Polymers 2022, 14, 769. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Soy hull as an adsorbent source in processing soy oil. J. Am. Oil Chem. Soc. 1997, 74, 685–692. [Google Scholar] [CrossRef]
- Bendahou, A.; Hajlane, A.; Dufresne, A.; Boufi, S.; Kaddami, H. Esterification and amidation for grafting long aliphatic chains on to cellulose nanocrystals: A comparative study. Res. Chem. Intermed. 2015, 41, 4293–4310. [Google Scholar] [CrossRef]
- Sim, K.; Youn, H.J.; Jo, Y. Surface modification of cellulose nanofibrils by carboxymethylation and tempo-mediated oxidation. Palpu Chongi Gisul/J. Korea Tech. Assoc. Pulp Pap. Ind. 2015, 47, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Wang, T.; Li, B. Preparation of a hydroxyethyl-titanium dioxide-carboxymethyl cellulose hydrogel cage and its effect on the removal of methylene blue. J. Appl. Polym. Sci. 2017, 134, 44925. [Google Scholar] [CrossRef]
- Thomas, M.; Naikoo, G.A.; Sheikh, M.U.D.; Bano, M.; Khan, F. Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J. Photochem. Photobiol. A Chem. 2016, 327, 33–43. [Google Scholar] [CrossRef]
- Jiao, Y.; Wan, C.; Li, J. Hydrothermal synthesis of SnO2-ZnO aggregates in cellulose aerogels for photocatalytic degradation of rhodamine b. Cellul. Chem. Technol. 2018, 52, 481–491. [Google Scholar]
- Hu, Y.; Li, N.; Yue, P.; Chen, G.; Hao, X.; Bian, J.; Peng, F. Highly antibacterial hydrogels prepared from amino cellulose, dialdehyde xylan, and Ag nanoparticles by a green reduction method. Cellulose 2022, 29, 1055–1067. [Google Scholar] [CrossRef]
- Obst, M.; Heinze, T. Simple synthesis of reactive and nanostructure forming hydrophobic amino cellulose derivatives. Macromol. Mater. Eng. 2016, 301, 65–70. [Google Scholar] [CrossRef]
- Ansari, M.M.; Ahmad, A.; Kumar, A.; Alam, P.; Khan, T.H.; Jayamurugan, G.; Raza, S.S.; Khan, R. Aminocellulose-grafted-polycaprolactone coated gelatin nanoparticles alleviate inflammation in rheumatoid arthritis: A combinational therapeutic approach. Carbohydr. Polym. 2021, 258, 117600. [Google Scholar] [CrossRef]
- Stöhr, M.; Sadhukhan, M.; Al-Hamdani, Y.S.; Hermann, J.; Tkatchenko, A. Coulomb interactions between dipolar quantum fluctuations in van der waals bound molecules and materials. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Dai, X.; Tao, T. Rapid photocatalytic degradation of pollutant from water under UV and sunlight via cellulose nanofiber aerogel wrapped by TiO2. J. Nanomater. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Habibi, S.; Jamshidi, M. Synthesis of TiO2 nanoparticles coated on cellulose nanofibers with different morphologies: Effect of the template and sol-gel parameters. Mater. Sci. Semicond. Process. 2020, 109, 104927. [Google Scholar] [CrossRef]
- Qin, C.C.; Li, S.J.; Jiang, G.Q.; Cao, J.; Guo, Y.L.; Li, J.W.; Zhang, B.; Han, S.S. Preparation of Flower-like ZnO Nanoparticles in a Cellulose Hydrogel Microreactor. BioResources 2017, 12, 3182–3191. [Google Scholar] [CrossRef] [Green Version]
- Sabbaghan, M.; Argyropoulos, D.S. Synthesis and characterization of nano fibrillated cellulose/Cu2O films; micro and nano particle nucleation effects. Carbohydr. Polym. 2018, 197, 614–622. [Google Scholar] [CrossRef]
- Qi, X.; Guan, Y.; Chen, G.; Zhang, B.; Ren, J.; Peng, F.; Sun, R. A non-covalent strategy for montmorillonite/xylose self-healing hydrogels. RSC Adv. 2015, 5, 41006–41012. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, L.; Du, X.; Fan, Z.; Wang, L.; Sun, W.; Cheng, Y.; Zhu, Y.; Chen, C. Reversible physical crosslinking strategy with optimal temperature for 3D bioprinting of human chondrocyte-laden gelatin methacryloyl bioink. J. Biomater. Appl. 2018, 33, 609–618. [Google Scholar] [CrossRef]
- Jo, S.; Oh, Y.; Park, S.; Kan, E.; Lee, S.H. Cellulose/carrageenan/TiO2 nanocomposite for adsorption and photodegradation of cationic dye. Biotechnol. Bioprocess Eng. 2017, 22, 734–738. [Google Scholar] [CrossRef]
- Su, X.; Liao, Q.; Liu, L.; Meng, R.; Qian, Z.; Gao, H.; Yao, J. Cu2O nanoparticle -functionalized cellulose-based aerogel as high-performance visible-light photocatalyst. Cellulose 2017, 24, 1017–1029. [Google Scholar] [CrossRef]
- Volokhova, A.A.; Kudryavtseva, V.L.; Spiridonova, T.I.; Kolesnik, I.; Goreninskii, S.I.; Sazonov, R.V.; Remnev, G.E.; Tverdokhlebov, S.I. Controlled drug release from electrospun PCL non-woven scaffolds via multi-layering and e-beam treatment. Mater. Today Commun. 2021, 26, 102134. [Google Scholar] [CrossRef]
- Ishak, W.H.W.; Jia, O.Y.; Ahmad, I. pH-responsive gamma-irradiated poly(acrylic acid)-cellulose-nanocrystal-reinforced hydrogels. Polymers 2020, 12, 1932. [Google Scholar] [CrossRef]
- Liu, G.; Li, T.T.; Song, X.F.; Yang, J.Y.; Qin, J.T.; Zhang, F.F.; Wang, Z.X.; Chen, H.G.; Wu, M.H.; Li, Y.S. Thermally driven characteristic and highly photocatalytic activity based on N-isopropyl acrylamide/high-substituted hydroxypropyl cellulose/g-C3N4 hydrogel by electron beam pre-radiation method. J. Thermoplast. Compos. Mater. 2020, 33, 089270572094421. [Google Scholar] [CrossRef]
- Mhsin, I.; Maria, Z. Titanium dioxide nanostructures as efficient photocatalyst: Progress, challenges and perspective. Int. J. Energy Res. 2021, 45, 3569–3589. [Google Scholar]
- Dearfield, K.L.; Harrington-Brock, K.; Doerr, C.L.; Rabinowitz, J.R.; Moore, M.M. Genotoxicity in mouse lymphoma cells of chemicals capable of Michael addition. Mutagenesis 1991, 6, 519–525. [Google Scholar] [CrossRef]
- Marcı, G.; Mele, G.; Palmisano, L.; Pulito, P.; Sannino, A. Environmentally sustainable production of cellulose-based superabsorbent hydrogels. Green Chem. 2006, 8, 439–444. [Google Scholar]
- Melone, L.; Altomare, L.; Alfieri, I.; Lorenzi, A.; De Nardo, L.; Punta, C. Ceramic aerogels from TEMPO-oxidized cellulose nanofibre templates: Synthesis, characterization, and photocatalytic properties. J. Photochem. Photobiol. A Chem. 2013, 261, 53–60. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Chen, L.; Cai, J.; Zhang, L. Facile construction of cellulose nanocomposite aerogel containing TiO2 nanoparticles with high content and small size and their applications. Cellulose 2017, 24, 2229–2240. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, X.; Wu, Q.; Han, J.; Jiang, J. Highly recyclable and super-tough hydrogel mediated by dual-functional TiO2 nanoparticles toward efficient photodegradation of organic water pollutants. J. Colloid Interface Sci. 2020, 564, 99–112. [Google Scholar] [CrossRef]
- Rahman, K.U.; Ferreira-Neto, E.P.; Rahman, G.U.; Parveen, R.; Monteiro, A.S.; Rahman, G.; Van Le, Q.; Domeneguetti, R.R.; Ribeiro, S.J.L.; Ullah, S. Flexible bacterial cellulose-based BC-SiO2-TiO2-Ag membranes with self-cleaning, photocatalytic, antibacterial and UV-shielding properties as a potential multifunctional material for combating infections and environmental applications. J. Environ. Chem. Eng. 2021, 9, 104708. [Google Scholar] [CrossRef]
- Li, K.; Zhang, X.; Qin, Y.; Li, Y. Construction of the Cellulose Nanofibers (CNFs) Aerogel Loading TiO2 NPs and Its Application in Disposal of Organic Pollutants. Polymers 2021, 13, 1841. [Google Scholar] [CrossRef]
- Hasanpour, M.; Motahari, S.; Jing, D.; Hatami, M. Investigation of the Different Morphologies of Zinc Oxide (ZnO) in Cellulose/ZnO Hybrid Aerogel on the Photocatalytic Degradation Efficiency of Methyl Orange. Top. Catal. 2021, 64, 1–14. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Cheng, Q.; Lv, F.; Chang, C.; Zhang, L. Construction of β-FeOOH@tunicate cellulose nanocomposite hydrogels and their highly efficient photocatalytic properties. Carbohydr. Polym. 2019, 229, 115470. [Google Scholar] [CrossRef]
- Zhu, Z.; Qu, J.; Hao, S.; Han, S.; Jia, K.; Yu, Z. Alpha-Fe2O3 Nanodisk/Bacterial Cellulose Hybrid Membranes as High-Performance Sulfate-Radical-Based Visible Light Photocatalysts under Stirring/Flowing States. ACS Appl. Mater. Interfaces 2018, 10, 30670–30679. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Chen, Z.; Qi, H. A novel cellulose-derived carbon aerogel@Na2Ti3O7 composite for efficient photocatalytic degradation of methylene blue. J. Appl. Polym. Sci. 2021, 138, 5134. [Google Scholar] [CrossRef]
- Hoa, N.T.; Tien, N.T.; Phong, L.H.; Tai, V.V.; Hung, N.V.; Nui, P.X. Photocatalytic activity of Ag-Ag3PO4/Cellulose aerogel composite for degradation of dye pollutants under visible light irradiation. Vietnam. J. Catal. Adsorpt. 2021, 10, 6–17. [Google Scholar]
- Wang, L.; Chen, S.; Wu, P.; Wu, K.; Wu, J.; Meng, G.; Hou, J.; Liu, Z.; Guo, X. Enhanced optical absorption and pollutant adsorption for photocatalytic performance of three-dimensional porous cellulose aerogel with BiVO4 and PANI. J. Mater. Res. 2020, 35, 1316–1328. [Google Scholar] [CrossRef]
- Ferreira-Neto, E.P.; Ullah, S.; Silva, T.C.A.; Domeneguetti, R.R.; Perrissinotto, A.P.; Vicente, F.S.; Rodrigues-Filho, U.P.; Ribeiro, S.J.L. Bacterial Nanocellulose/MoS2 Hybrid Aerogels as Bifunctional Adsorbent/Photocatalyst Membranes for in-Flow Water Decontamination. ACS Appl. Mater. Interfaces 2020, 12, 41627–41643. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B: Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Qian, X.; Xu, Y.; Yue, X.; Wang, C.; Liu, M.; Duan, C.; Xu, Y.; Zhu, C.; Dai, L. Microwave-assisted solvothermal in-situ synthesis of CdS nanoparticles on bacterial cellulose matrix for photocatalytic application. Cellulose 2020, 27, 5939–5954. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, J.; Hao, C.; Mei, S.; Yang, J.; Wang, X.; Zhao, R.; Zhai, X.; Liu, Y. Easily recoverable CdxZn1-xS-Gel photocatalyst with a tunable band structure for efficient and stable H2 production mediated by visible light. Cellulose 2018, 25, 167–177. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Heidarpour, H.; Golizadeh, M.; Padervand, M.; Karimi, A.; Vossoughi, M.; Tavakoli, M.H. In-situ formation and entrapment of Ag/AgCl photocatalyst inside cross-linked carboxymethyl cellulose beads: A novel photoactive hydrogel for visible-light-induced photocatalysis. J. Photochem. Photobiol. A Chem. 2020, 398, 112559. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, S.; Gao, Y.; Rony, A.H.; Fan, M.; Tan, G. Surface modification of porous g-C3N4 materials using a waste product for enhanced photocatalytic performance under visible light. Green Chem. 2019, 21, 5934–5944. [Google Scholar] [CrossRef]
- Chen, S.; Lu, W.; Han, J.; Zhong, H.; Xu, T.; Wang, G.; Chen, W. Robust three-dimensional g-C3N4@cellulose aerogel enhanced by cross-linked polyester fibers for simultaneous removal of hexavalent chromium and antibiotics. Chem. Eng. J. 2019, 359, 119–129. [Google Scholar] [CrossRef]
- Bai, W.; Yang, X.; Du, X.; Qian, Z.; Zhang, Y.; Liu, L.; Yao, J. Robust and recyclable macroscopic g-C3N4/cellulose hybrid photocatalysts with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2019, 504, 144179. [Google Scholar] [CrossRef]
- Qi, H.; Ji, X.; Shi, C.; Ma, R.; Huang, Z.; Guo, M.; Li, J.; Guo, Z. Bio-templated 3D porous graphitic carbon nitride hybrid aerogel with enhanced charge carrier separation for efficient removal of hazardous organic pollutants. J. Colloid Interface Sci. 2019, 556, 366–375. [Google Scholar] [CrossRef]
- Yao, M.; Wang, Z.; Liu, Y.; Yang, G.; Chen, J. Preparation of dialdehyde cellulose graftead graphene oxide composite and its adsorption behavior for heavy metals from aqueous solution. Carbohydr. Polym. 2019, 212, 345–351. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.; Wang, X.; Yang, G.; Liu, Y. Adsorption-photocatalysis performance of polyaniline/dicarboxyl acid cellulose@graphene oxide for dye removal. Int. J. Biol. Macromol. 2021, 182, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, L.; Li, J.; Rong, S.; Jiang, J.; Liu, S. Photocatalytic Degradation of Tetracycline by a Novel (CMC)/MIL-101(Fe)/β-CDP Composite Hydrogel. Front. Chem. 2021, 8, 593730. [Google Scholar] [CrossRef] [PubMed]
- Levec, J.; Pintar, A. Catalytic wet-air oxidation processes: A review. Catal. Today 2007, 124, 172–184. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, K.; Yang, W.; Chen, G.; Zhang, X.; Zhao, Y.; Liu, L.; Chen, M. Efficient removal of Cd2+ ion from water by calcium alginate hydrogel filtration membrane. Water Sci. Technol. 2017, 75, 2322–2330. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, P.; Zhang, L.; Zhong, Y.; Sui, X.; Wang, B.; Ma, Y.; Feng, X.; Xu, H.; Mao, Z. A recyclable 3D g-C3N4 based nanocellulose aerogel composite for photodegradation of organic pollutants. Cellulose 2021, 28, 3531–3547. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Q.F.; Li, J.; Liu, Y. Fabrication, Characterization and Photocatalytic Activity of TiO2/Cellulose Composite Aerogel. Key Eng. Mater. 2014, 609–610, 542–546. [Google Scholar] [CrossRef]
- Tang, L.; Fu, T.; Li, M.; Li, L. Facile synthesis of Ag@AgCl-contained cellulose hydrogels and their application. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 618–623. [Google Scholar] [CrossRef]
- Hasanpour, M.; Motahari, S.; Jing, D.; Hatami, M. Statistical analysis and optimization of photodegradation efficiency of methyl orange from aqueous solution using cellulose/zinc oxide hybrid aerogel by response surface methodology (RSM). Arab. J. Chem. 2021, 14, 103401. [Google Scholar] [CrossRef]
- Brandes, R.; Trindade, E.C.A.; Vanin, D.F.; Vargas, V.M.M.; Carminatti, C.A.; AL-Qureshi, H.A.; Recouvreux, D.O. Spherical Bacterial Cellulose/TiO2 Nanocomposite with Potential Application in Contaminants Removal from Wastewater by Photocatalysis. Fibers Polym. 2018, 19, 1861–1868. [Google Scholar] [CrossRef]
- Jiao, Y.; Wan, C.; Li, J. Room-temperature embedment of anatase titania nanoparticles into porous cellulose aerogels. Appl. Phys. 2015, 120, 341–347. [Google Scholar] [CrossRef]
- Li, M.; Qiu, J.; Xu, J.; Yao, J. Cellulose/TiO2-based carbonaceous composite film and aerogel for high-efficient photocatalysis under visible light. Ind. Eng. Chem. Res. 2020, 59, 13997–14003. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Y.; Fang, C.; Shi, J.; Chen, J.; Han, H. Novel and multifunctional adsorbent fabricated by Zeolitic imidazolate framworks-8 and waste cigarette filters for wastewater treatment: Effective adsorption and photocatalysis. J. Solid State Chem. 2021, 299, 122190. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Sabidi, S.; Ohno, T.; Maeda, T.; Andou, Y. Cu2O/TiO2 decorated on cellulose nanofiber/reduced graphene hydrogel for enhanced photocatalytic activity and its antibacterial applications. Chemosphere 2022, 286, 131731. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, Z.; Pan, J.; Gong, W.; Liao, Q.; Liu, L.; Yao, J. High photocatalytic activity of Cu@Cu2O/RGO/cellulose hybrid aerogels as reusable catalysts with enhanced mass and electron transfer. React. Funct. Polym. 2019, 138, 79–87. [Google Scholar]
- Qiu, J.; Fan, P.; Yue, C.; Liu, F.; Li, A. Multi-networked nanofibrous aerogel supported by heterojunction photocatalysts with excellent dispersion and stability for photocatalysis. J. Mater. Chem. A 2019, 7, 7053–7064. [Google Scholar] [CrossRef]
- Xue, C.; Zheng, C.; Zhao, Q.; Sun, S. Occurrence of antibiotics and antibiotic resistance genes in cultured prawns from rice-prawn co-culture and prawn monoculture systems in China. Sci. Total Environ. 2022, 806, 150307. [Google Scholar] [CrossRef]
- Amaly, N.; EL-Moghazy, A.Y.; Nitin, N.; Sun, G.; Pandey, P.K. Synergistic adsorption-photocatalytic degradation of tetracycline by microcrystalline cellulose composite aerogel dopped with montmorillonite hosted methylene blue. Chem. Eng. J. 2022, 430, 133077. [Google Scholar] [CrossRef]
- Yue, Y.; Shen, S.; Cheng, W.; Han, G.; Wu, Q.; Jiang, J. Construction of mechanically robust and recyclable photocatalytic hydrogel based on nanocellulose-supported CdS/MoS2/Montmorillonite hybrid for antibiotic degradation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128035. [Google Scholar] [CrossRef]
- Montgomery, M.A.; Elimelech, M. Water and Sanitation in Developing Countries: Including Health in the Equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Hu, Y.; Chen, A.; Zhou, L.; Gao, H.; Liu, Y.; Liu, S. Study on Photocatalytic Antibacterial and Sustained-Release Properties of Cellulose/TiO2/β-CD Composite Hydrogel. J. Nanomater. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Hong, M.; Zhang, L.; Feng, X.; Shi, M.; Hu, W.; Mu, S. Defective RuO2/TiO2 nano-heterostructure advances hydrogen production by Electrochemical Water Splitting. Chem. Eng. J. 2021, 431, 134072. [Google Scholar] [CrossRef]
- Zhong, D.; Li, J.; Ma, W.; Xin, H. Magnetite nanoparticles enhanced glucose anaerobic fermentation for bio-hydrogen production using an expanded granular sludge bed (EGSB) reactor. Int. J. Hydrog. Energy 2020, 45, 10664–10672. [Google Scholar] [CrossRef]
- Kang, H.; Gao, J.; Xie, M.; Sun, Y.; Wu, F.; Gao, C.; Liu, Y.; Qiu, H. Carboxymethyl cellulose gel membrane loaded with nanoparticle photocatalysts for hydrogen production. Int. J. Hydrog. Energy 2019, 44, 13011–13021. [Google Scholar] [CrossRef]
- Xie, Y.; Pan, Y.; Cai, P. Cellulose-based antimicrobial films incroporated with ZnO nanopillars on surface as biodegradable and antimicrobial packaging. Food Chem. 2021, 368, 130784. [Google Scholar] [CrossRef] [PubMed]



| Categories | Photocatalysts | Hydrogel Materials | Characteristics | Preparation Methods | Specific Surface Area/m2g−1 | References |
|---|---|---|---|---|---|---|
| Metal Oxide Semiconductor Composites | ZnO | PAM/CMC/DDM | Suspended hydrogels, adsorb heavy metal ions, and degrade dyes efficiently | Mechanical foaming and in situ polymerization | - | Zhao et al. 2021 |
| Cellulose | Dispersion framework for nanomaterials | Physical crosslinking | - | Jiao et al. 2018 | ||
| Bamboo fiber | High specific surface area | Chemical crosslinking | 39.18 | Qin et al. 2017 | ||
| Cu2O | Cellulose/AA/AM | High adsorption | Chemical crosslinking | 89.56 | Su et al. 2017 | |
| TiO2 | CMCNa/HEC | Superabsorbent, biodegradable, and photocatalytic degradation crosslinker | Chemical crosslinking | - | Marcı et al. 2006 | |
| Cotton cellulose | High temperature resistant | Physical crosslinking | 6.10 | Melone et al. 2013 | ||
| α-Cellulose | TiO2 in situ generators, excellent strength and good toughness | Chemical crosslinking | 550 | Wang et al. 2017 | ||
| TOCNs/PAM | Super-tough | Chemical crosslinking | - | Yue et al. 2020 | ||
| BC | Self-cleaning, antibacterial, and UV shielding | Chemical crosslinking | - | Rahman et al. 2021 | ||
| CNFs | Good adsorption, photocatalytic degradation ability, low density, and easy recovery | Chemical crosslinking | 330 | Li et al. 2021 | ||
| Na2Ti3O7 | Sisal cellulose | High specific surface area | Physical crosslinking | 248.93 | Liu et al. 2021 | |
| Metal sulfide (chloride) semiconductor composites | MoS2 | BC | Bifunctional adsorbent/photocatalyst membranes | Chemical crosslinking | 137 | Ferreira-Neto et al. 2022 |
| CdS | Straw cellulose | Green recyclable | Chemical crosslinking | - | Qian et al. 2020 | |
| CdxZn1-xS | CMC | High yield of hydrogen, good stability, easy recovery | Chemical crosslinking | - | Wu et al. 2018 | |
| Ag/AgCl | CMC | Hydrogel beads, photocatalytic degradation of RhB | Chemical crosslinking | - | Heidarpour et al. 2020 | |
| Organic semiconductor composites | g-C3N4 | Polyester fiber/cotton wool | High specific surface area, impact resistant | Chemical crosslinking | - | Chen et al. 2019 |
| Cotton linter | Enhanced carrier separation | - | - | Bai et al. 2019; Yao et al. 2019 | ||
| CMC/β-Cyclodextrin | ||||||
| GO | MCC | Adsorption–photocatalytic synergy | Chemical crosslinking | 48.6 | Liu et al. 2021 | |
| MIL-100(Fe) | CMC/β-Cyclodextrin | Good water retainability | Chemical crosslinking | - | Zhang et al. 2021 |
| Ag/AgCl@Ag-CMC | AgCl@Fe-CMC | |||||||
|---|---|---|---|---|---|---|---|---|
| Catalyst Dosage | 1 (g/L) | 2 (g/L) | 4 (g/L) | 6 (g/L) | 1 (g/L) | 2 (g/L) | 4 (g/L) | 6 (g/L) |
| Kapp | 0.0101 | 0.0223 | 0.0517 | 0.0711 | 0.0073 | 0.0152 | 0.0304 | 0.0395 |
| R2 | 0.98 | 0.99 | 0.95 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 |
| RhB concentration | 10 (ppm) | 15 (ppm) | 20 (ppm) | 25 (ppm) | 10 (ppm) | 15 (ppm) | 20 (ppm) | 25 (ppm) |
| Kapp | 0.0517 | 0.0318 | 0.0233 | 0.0141 | 0.0304 | 0.0224 | 0.0170 | 0.0103 |
| R2 | 0.95 | 0.99 | 0.97 | 0.99 | 0.99 | 0.99 | 0.98 | 0.99 |
| pH | 4 | 7 | 9 | 4 | 7 | 9 | ||
| Kapp | 0.0295 | 0.0517 | 0.0673 | 0.0198 | 0.0304 | 0.0352 | ||
| R2 | 0.99 | 0.95 | 0.99 | 0.99 | 0.99 | 0.95 | ||
| Dye | Catalysts | Dye Concentration (mg/L) | Time (min) | Degradation (%) | References |
|---|---|---|---|---|---|
| MO | TiO2-TOCNs-PAM | 10 | 90 | 97.3 | Yue et al. 2020 |
| CA/ZnO | 20 | 120 | 94.78 | Hasanpour et al. 2021 | |
| g-C3N4 Cellulose aerogel | 20 | 180 | 99 | Ma et al. 2021 | |
| Ag@AgCl-contained cellulose hydrogel | 10 | 70 | 93 | Tang et al. 2018 | |
| Cu2O/TiO2/CNF/rGH | 20 | 120 | 85.62 | Zheng et al. 2022 | |
| Cu@Cu2O/RGO/cellulose hybrid aerogel | 10 | 120 | 92.8 | Du et al. 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Liu, D.; Song, X.; Zhao, Y.; Wang, Y.; Rao, L.; Fu, L.; Wang, Z.; Yang, X.; Li, Y.; et al. Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications. Gels 2022, 8, 270. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050270
Yang J, Liu D, Song X, Zhao Y, Wang Y, Rao L, Fu L, Wang Z, Yang X, Li Y, et al. Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications. Gels. 2022; 8(5):270. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050270
Chicago/Turabian StyleYang, Jinyu, Dongliang Liu, Xiaofang Song, Yuan Zhao, Yayang Wang, Lu Rao, Lili Fu, Zhijun Wang, Xiaojie Yang, Yuesheng Li, and et al. 2022. "Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications" Gels 8, no. 5: 270. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050270