A Plant-Based Animal Fat Analog Produced by an Emulsion Gel of Alginate and Pea Protein
Abstract
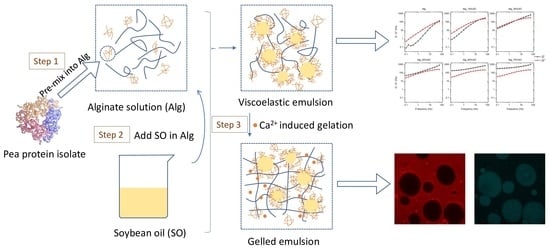
:1. Introduction
2. Results and Discussion
2.1. Rheology
2.2. Color Measurement of Gelled Emulsions
2.3. Confocal Laser Scanning Microscopy
2.4. Differential Scanning Calorimetry (DSC)
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. Surface Extractable Lipid
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Pea Protein Isolate Purity Determination
4.3. Preparation of the Gel
4.4. Rheology
4.5. Colorimetry
4.6. Confocal Laser Scanning Microscopy
4.7. Differential Scanning Calorimetry (DSC)
4.8. Fourier Transform Infrared (FTIR) Spectroscopy
4.9. Surface Extractable Fat
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Improved Functional Properties of Meat Analogs by Laccase Catalyzed Protein and Pectin Crosslinks. Sci. Rep. 2021, 11, 16631. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Trivedi, N.; Enamala, M.K.; Kuppam, C.; Parikh, P.; Nikolova, M.P.; Chavali, M. Plant-Based Meat Analogue (PBMA) as a Sustainable Food: A Concise Review. Eur. Food Res. Technol. 2021, 247, 2499–2526. [Google Scholar] [CrossRef]
- Papier, K.; Fensom, G.K.; Knuppel, A.; Appleby, P.N.; Tong, T.Y.N.; Schmidt, J.A.; Travis, R.C.; Key, T.J.; Perez-Cornago, A. Meat Consumption and Risk of 25 Common Conditions: Outcome-Wide Analyses in 475,000 Men and Women in the UK Biobank Study. BMC Med. 2021, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.W.; Bonham, M.P.; Fearon, A.M. Red Meat Consumption: An Overview of the Risks and Benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Chapter 6-Plant-Based Meat Analogues. In Sustainable Meat Production and Processing; Galanakis, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 103–126. ISBN 978-0-12-814874-7. [Google Scholar]
- Schwingshackl, L.; Zähringer, J.; Beyerbach, J.; Werner, S.W.; Heseker, H.; Koletzko, B.; Meerpohl, J.J. Total Dietary Fat Intake, Fat Quality, and Health Outcomes: A Scoping Review of Systematic Reviews of Prospective Studies. Ann. Nutr. Metab. 2021, 77, 4–15. [Google Scholar] [CrossRef]
- Krauss, R.M.; Kris-Etherton, P.M. Public Health Guidelines Should Recommend Reducing Saturated Fat Consumption as Much as Possible: NO. Am. J. Clin. Nutr. 2020, 112, 19–24. [Google Scholar] [CrossRef]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of Oleogelation on Physical Properties and Oxidative Stability of Camellia Oil-Based Oleogels and Oleogel Emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef]
- Papadaki, A.; Cipolatti, E.P.; Aguieiras, E.C.G.; Cerqueira Pinto, M.C.; Kopsahelis, N.; Freire, D.M.G.; Mandala, I.; Koutinas, A.A. Development of Microbial Oil Wax-Based Oleogel with Potential Application in Food Formulations. Food Bioprocess. Technol. 2019, 12, 899–909. [Google Scholar] [CrossRef]
- Barroso, N.G.; Santos, M.A.S.; Okuro, P.K.; Cunha, R.L. Composition and Process Approaches That Underpin the Mechanical Properties of Oleogels. J. Am. Oil Chem. Soc. 2022, 99, 971–984. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, L.; Zhang, Y.; Li, H.; Zhao, D.; Cao, J.; Liu, X. Application of Emulsion Gels as Fat Substitutes in Meat Products. Foods 2022, 11, 1950. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Yuan, F.; Gao, Y.; Mao, L. Emulsion Gels with Different Proteins at the Interface: Structures and Delivery Functionality. Food Hydrocoll. 2021, 116, 106637. [Google Scholar] [CrossRef]
- Hu, X.; McClements, D.J. Construction of Plant-Based Adipose Tissue Using High Internal Phase Emulsions and Emulsion Gels. Innov. Food Sci. Emerg. Technol. 2022, 78, 103016. [Google Scholar] [CrossRef]
- Maiti, S.; Kumari, L. Smart Nanopolysaccharides for the Delivery of Bioactives. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Grumezescu, A.M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 67–94. ISBN 978-0-323-47347-7. [Google Scholar]
- Loureiro dos Santos, L.A. Natural Polymeric Biomaterials: Processing and Properties. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-803581-8. [Google Scholar]
- Ong, W.-D.; Tey, B.-T.; Quek, S.Y.; Tang, S.-Y.; Chan, E.-S. Alginate-Based Emulsion Template Containing High Oil Loading Stabilized by Nonionic Surfactants. J. Food Sci. 2015, 80, E93–E100. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Yong, H.-I.; Jung, S.; Kim, Y.-B.; Choi, Y.-S. Effects of Replacing Pork Fat with Grape Seed Oil and Gelatine/Alginate for Meat Emulsions. Meat Sci. 2020, 163, 108079. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Geng, S.; Zhen, S.; Lv, X.; Liu, B. Fabrication and Characterization of Oil-in-Water Emulsions Stabilized by Whey Protein Isolate/Phloridzin/Sodium Alginate Ternary Complex. Food Hydrocoll. 2022, 129, 107625. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Maidannyk, V.; Miao, S. Effect of Concentrations of Alginate, Soy Protein Isolate and Sunflower Oil on Water Loss, Shrinkage, Elastic and Structural Properties of Alginate-Based Emulsion Gel Beads during Gelation. Food Hydrocoll. 2020, 108, 105998. [Google Scholar] [CrossRef]
- Sridharan, S.; Meinders, M.B.J.; Bitter, J.H.; Nikiforidis, C.V. On the Emulsifying Properties of Self-Assembled Pea Protein Particles. Langmuir 2020, 36, 12221–12229. [Google Scholar] [CrossRef]
- Kornet, R.; Yang, J.; Venema, P.; van der Linden, E.; Sagis, L.M.C. Optimizing Pea Protein Fractionation to Yield Protein Fractions with a High Foaming and Emulsifying Capacity. Food Hydrocoll. 2022, 126, 107456. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-Moisture Meat Analogues Produced from Yellow Pea and Faba Bean Protein Isolates/Concentrate: Effect of Raw Material Composition and Extrusion Parameters on Texture Properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef]
- Mason, T.G. New Fundamental Concepts in Emulsion Rheology. Curr. Opin. Colloid Interface Sci. 1999, 4, 231–238. [Google Scholar] [CrossRef]
- Yang, M.; Liu, F.; Tang, C.-H. Properties and Microstructure of Transglutaminase-Set Soy Protein-Stabilized Emulsion Gels. Food Res. Int. 2013, 52, 409–418. [Google Scholar] [CrossRef]
- Martínez, S.; Espert, M.; Salvador, A.; Sanz, T. The Role of Oil Concentration on the Rheological Properties, Microstructure, and in Vitro Digestion of Cellulose Ether Emulsions. Food Hydrocoll. 2022, 131, 107793. [Google Scholar] [CrossRef]
- Liang, S.; Wang, L. A Natural Antibacterial-Antioxidant Film from Soy Protein Isolate Incorporated with Cortex Phellodendron Extract. Polymers 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, L.; Abdolmaleki, K.; Nayebzadeh, K.; Bahmaei, M. Characterization of Sodium Caseinate/Hydroxypropyl Methylcellulose Concentrated Emulsions: Effect of Mixing Ratio, Concentration and Wax Addition. Int. J. Biol. Macromol. 2019, 128, 796–803. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S. Effects of Protein Concentration and Oil-Phase Volume Fraction on the Stability and Rheology of Menhaden Oil-in-Water Emulsions Stabilized by Whey Protein Isolate with Xanthan Gum. Food Hydrocoll. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Synergistic Effects of Laccase and Pectin on the Color Changes and Functional Properties of Meat Analogs Containing Beet Red Pigment. Sci. Rep. 2022, 12, 1168. [Google Scholar] [CrossRef]
- Bulkaini, B.; Dahlanuddin, D.; Ariana, T.; Kisworo, D.; Maskur, M.; Mastur, M. Marbling Score, Cholesterol, and Physical–Chemical Content of Male Bali Beef Fed Fermented Pineapple Peel. J. Adv. Vet. Anim. Res. 2022, 9, 419. [Google Scholar] [CrossRef]
- Sharma, G.; Bala, R. Digital Color Imaging Handbook; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1351835971. [Google Scholar]
- Huang, L.; Liu, J.; Addy, M.; Ding, B.; Cheng, Y.; Peng, P.; Wang, Y.; Liu, Y.; Chen, P.; Ruan, R. Physicochemical and Emulsifying Properties of Orange Fibers Stabilized Oil-in-Water Emulsions. LWT 2020, 133, 110054. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Chen, C.; He, R.; Ju, X. Rapeseed Protein Nanogels as Novel Pickering Stabilizers for Oil-in-Water Emulsions. J. Agric. Food Chem. 2020, 68, 3607–3614. [Google Scholar] [CrossRef]
- Dron, S.M.; Paulis, M. Tracking Hydroplasticization by DSC: Movement of Water Domains Bound to Poly(Meth)Acrylates during Latex Film Formation. Polymers 2020, 12, 2500. [Google Scholar] [CrossRef]
- Talik, P.; Hubicka, U. The DSC Approach to Study Non-Freezing Water Contents of Hydrated Hydroxypropylcellulose (HPC). J. Therm. Anal. Calorim. 2018, 132, 445–451. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, Y.; Zhang, H.; Liu, Y. Investigation on Gelation Nucleation Kinetics of Waxy Crude Oil Emulsions by Their Thermal Behavior. J. Pet. Sci. Eng. 2019, 181, 106230. [Google Scholar] [CrossRef]
- Bayard, M.; Cansell, M.; Leal-Calderon, F. Crystallization of Emulsified Anhydrous Milk Fat: The Role of Confinement and of Minor Compounds. A DSC Study. Food Chem. 2022, 373, 131605. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.I.; Chong, P.S.; Stapley, A.G.F. Investigation of the Crystallization and Melting of the Tripalmitin/Triolein System via Hot Stage Microscopy, Differential Scanning Calorimetry, and Pulsed NMR. Cryst. Growth Des. 2017, 17, 3005–3016. [Google Scholar] [CrossRef]
- Wang, T.; Briggs, J.L. Rheological and Thermal Properties of Soybean Oils with Modified FA Compositions. J. Am. Oil Chem. Soc. 2002, 79, 831–836. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Carvajal, M.T.; Harris, M.T. Interactions between Bovine Serum Albumin and Alginate: An Evaluation of Alginate as Protein Carrier. J. Colloid Interface Sci. 2009, 332, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Bajas, D.; Vlase, G.; Mateescu, M.; Grad, O.A.; Bunoiu, M.; Vlase, T.; Avram, C. Formulation and Characterization of Alginate-Based Membranes for the Potential Transdermal Delivery of Methotrexate. Polymers 2021, 13, 161. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, S.; Zhang, Y.; Shi, L.; Ren, Z.; Hao, G.; Weng, W. Effects of Microfluidization Cycles on Physicochemical Properties of Soy Protein Isolate-Soy Oil Emulsion Films. Food Hydrocoll. 2022, 130, 107684. [Google Scholar] [CrossRef]
- Bao, L.; Bian, L.; Zhao, M.; Lei, J.; Wang, J. Synthesis and Self-Assembly Behavior of a Biodegradable and Sustainable Soybean Oil-Based Copolymer Nanomicelle. Nanoscale Res. Lett. 2014, 9, 391. [Google Scholar] [CrossRef]
- Zou, Y.; Xi, Y.; Pan, J.; Ijaz Ahmad, M.; Zhang, A.; Zhang, C.; Li, Y.; Zhang, H. Soy Oil and SPI Based-Oleogels Structuring with Glycerol Monolaurate by Emulsion-Templated Approach: Preparation, Characterization and Potential Application. Food Chem. 2022, 397, 133767. [Google Scholar] [CrossRef]
- Albano, K.M.; Cavallieri, Â.L.F.; Nicoletti, V.R. Electrostatic Interaction between Proteins and Polysaccharides: Physicochemical Aspects and Applications in Emulsion Stabilization. Food Rev. Int. 2019, 35, 54–89. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. FTIR Analysis of Proteins and Protein–Membrane Interactions-Lipid-Protein Interactions: Methods and Protocols; Kleinschmidt, J.H., Ed.; Springer: New York, NY, USA, 2019; pp. 281–325. ISBN 978-1-4939-9512-7. [Google Scholar]
- Branco, I.G.; Sen, K.; Rinaldi, C. Effect of Sodium Alginate and Different Types of Oil on the Physical Properties of Ultrasound-Assisted Nanoemulsions. Chem. Eng. Process. Process Intensif. 2020, 153, 107942. [Google Scholar] [CrossRef]
- Sosa-Herrera, M.G.; Lozano-Esquivel, I.E.; Ponce de León-Ramírez, Y.R.; Martínez-Padilla, L.P. Effect of Added Calcium Chloride on the Physicochemical and Rheological Properties of Aqueous Mixtures of Sodium Caseinate/Sodium Alginate and Respective Oil-in-Water Emulsions. Food Hydrocoll. 2012, 29, 175–184. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. Formation and Creaming Stability of Alginate/Micro-Gel Particle-Induced Gel-like Emulsions Stabilized by Soy Protein Isolate. Food Hydrocoll. 2021, 121, 107040. [Google Scholar] [CrossRef]
- Chen, D.; Campanella, O.H. Limited Enzymatic Hydrolysis Induced Pea Protein Gelation at Low Protein Concentration with Less Heat Requirement. Food Hydrocoll. 2022, 128, 107547. [Google Scholar] [CrossRef]
- Willett, S.A.; Akoh, C.C. Encapsulation of Menhaden Oil Structured Lipid Oleogels in Alginate Microparticles. LWT 2019, 116, 108566. [Google Scholar] [CrossRef]







| n | K | R2 | |
|---|---|---|---|
| Alg | 0.936 ± 0.004 | 1.043 ± 2.325 | 0.9997 |
| Alg_15%SO | 0.844 ± 0.015 | 6.272 ± 2.387 | 0.9956 |
| Alg_30%SO | 0.590 ± 0.017 | 25.421 ± 2.400 | 0.9882 |
| Alg_45%SO | 0.542 ± 0.007 | 33.083 ± 2.336 | 0.9981 |
| Alg_60%SO | 0.352 ± 0.010 | 65.902 ± 2.348 | 0.9906 |
| Alg_70%SO | 0.282 ± 0.010 | 92.449 ± 2.342 | 0.9875 |
| Sample | L | a | b | ΔE |
|---|---|---|---|---|
| Alg_15%SO | 50.26 ± 2.67 A | −0.17 ± 0.20 A | 0.85 ± 1.18 A | 28.82 ± 2.58 A |
| Alg_30%SO | 58.08 ± 1.17 B | −0.13 ± 0.25 A | 2.48 ± 0.25 AB | 20.9 ± 1.02 B |
| Alg_45%SO | 65.09 ± 1.11 C | −0.40 ± 0.20 A | 3.96 ± 0.10 BC | 13.92 ± 1.06 C |
| Alg_60%SO | 69.89 ± 1.97 D | −0.55 ± 0.04 A | 5.15 ± 0.27 CD | 9.28 ± 1.76 D |
| Alg_70%SO | 75.94 ± 3.77 E | −0.53 ± 0.51 A | 6.62 ± 0.98 D | 5.23 ± 1.75 E |
| Pea protein | 89.08 ± 0.23 F | 1.73 ± 0.05 B | 16.00 ± 0.31 F | 13.27 ± 0.15 C |
| Beef fat trimming | 77.72 ± 2.71 E | 2.28 ± 0.67 B | 9.16 ± 1.52 E | - |
| Sample | Ton | Tp1 | Tp2 |
|---|---|---|---|
| Alg_15%SO | −43.82 ± 0.21 A | −39.09 ± 0.14 A | −26.18 ± 0.14 A |
| Alg_30%SO | −43.96 ± 0.28 A | −39.19 ± 0.28 A | −26.33 ± 0.29 A |
| Alg_45%SO | −44.15 ± 0.18 A | −39.09 ± 0.11 A | −26.43 ± 0.27 A |
| Alg_60%SO | −43.74 ± 0.55 A | −39.10 ± 0.18 A | −26.46 ± 0.15 A |
| Alg_70%SO | −43.79 ± 0.23 A | −39.07 ± 0.23 A | −26.58 ± 0.21 A |
| SO | −44.18 ± 0.04 A | −39.09 ± 0.01 A | −26.28 ± 0.13 A |
| Sample Code | Alginate Solution (g) | Pea Protein (g) | SO (g) |
|---|---|---|---|
| Alg | 100 | 0 | 0 |
| Alg_15%SO | 85 | 0.15 | 15 |
| Alg_30%SO | 70 | 0.3 | 30 |
| Alg_45%SO | 55 | 0.45 | 45 |
| Alg_60%SO | 40 | 0.6 | 60 |
| Alg_70%SO | 30 | 0.7 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, C.; Campanella, O.H. A Plant-Based Animal Fat Analog Produced by an Emulsion Gel of Alginate and Pea Protein. Gels 2023, 9, 393. https://0-doi-org.brum.beds.ac.uk/10.3390/gels9050393
Teng C, Campanella OH. A Plant-Based Animal Fat Analog Produced by an Emulsion Gel of Alginate and Pea Protein. Gels. 2023; 9(5):393. https://0-doi-org.brum.beds.ac.uk/10.3390/gels9050393
Chicago/Turabian StyleTeng, Chong, and Osvaldo H. Campanella. 2023. "A Plant-Based Animal Fat Analog Produced by an Emulsion Gel of Alginate and Pea Protein" Gels 9, no. 5: 393. https://0-doi-org.brum.beds.ac.uk/10.3390/gels9050393