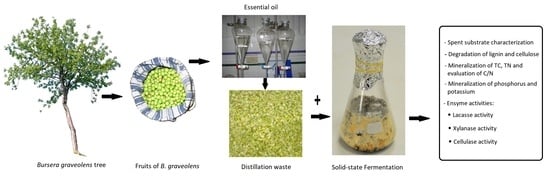
Biodegradation of Residues from the Palo Santo (Bursera graveolens) Essential Oil Extraction and Their Potential for Enzyme Production Using Native Xylaria Fungi from Southern Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of Fungi
2.2. Isolation, DNA Extraction, PCR and Fungal Sequencing
2.3. Preparation and Chemical Analysis of the Spent Substrate
2.4. Solid State Fermentation
2.5. Chemical Analysis and Quantification of BGR’s Degradation
2.6. Enzymatic Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Identification of Fungi
3.2. Spent Substrate Characterization
3.3. Efficiency of Degradation of the Three Fungi in the BGR’s
3.4. Mineralization of TC, TN and Evaluation of C/N Ratio
3.5. Mineralization of Phosphorus and Potassium
3.6. Enzyme Activities
3.6.1. Laccase Activity
3.6.2. Xylanase Activity
3.6.3. Cellulase Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weeks, A.; Tye, A. Phylogeography of palo santo trees (Bursera graveolens and Bursera malacophylla; Burseraceae) in the Galápagos archipelago. Bot. J. Linn. Soc. 2009, 161, 396–410. [Google Scholar] [CrossRef]
- Carrión-Paladines, V.; García-Ruiz, R. Floristic composition and structure of a deciduous dry forest from southern Ecuador: Diversity and aboveground carbon accumulation. Int. J. Curr. Res. Acad. Rev. 2016, 4, 154–169. [Google Scholar] [CrossRef]
- Yukawa, C.; Imayoshi, Y.; Iwabuchi, H.; Komemushi, S.; Sawabe, A. Chemical composition of three extracts of Bursera graveolens. Flavour Frag. J. 2006, 21, 234–238. [Google Scholar] [CrossRef]
- Alonso-Castro, A.; Villareal, M.; Salazar-Olivo, L.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Paladines, V.; Fries, A.; Gómez-Muñoz, B.; García-Ruiz, R. Agrochemical characterization of vermicomposts produced from residues of Palo Santo (Bursera graveolens) essential oil extraction. Waste Manag. 2016, 58, 135–143. [Google Scholar]
- Schmid, A.; Hollmann, F.; Park, J.B.; Bühler, B. The use of enzymes in the chemical industry in Europe. Curr. Opin. Biotechnol. 2002, 13, 359–366. [Google Scholar] [CrossRef]
- Johannes, T.; Zhao, H. Directed evolution of enzymes and biosynthetic pathways. Curr. Opin. Microbiol. 2006, 9, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech. 2016, 6, 3–15. [Google Scholar] [CrossRef]
- Demain, A.; Adrio, J. Contributions of microorganisms to industrial biology. Mol. Biotechnol. 2008, 38, 41–55. [Google Scholar] [CrossRef]
- Trivedi, N.; Reddy, C.R.K.; Radulovich, R.; Jha, B. Solid state fermentation (SSF)-derived cellulase for saccharification of the green seaweed Ulva for bioethanol production. Algal Res. 2015, 9, 48–54. [Google Scholar] [CrossRef]
- Araújo, R.; Casal, M.; Cavaco-Paulo, A. Application of enzymes for textile fibres processing. Biocatal. Biotransform. 2008, 26, 332–349. [Google Scholar] [Green Version]
- Rodríguez Couto, S.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Mainardi, P.; Feitosa, V.; Brenelli de Paiva, L. Laccase production in biorreactor scale under saline condition by the marine-derived basidiomycete Peniophora sp. CBMAI 1063. Fungal Biol. 2018, 122, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; del Río, J.; Martínez, A. Microbial and enzymatic control of pitch in the pulp and paper industry. Appl. Microbiol. Biotechnol. 2009, 82, 1005–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trierveiler-Pereira, L.; Romero, A.; Baltazar, J.; Loguercio-Leite, C. Addition to the knowledge of Xylaria (Xylariaceae, Ascomycota) in Santa Catarina, Southern Brazil. Mycotaxon 2008, 107, 139–156. [Google Scholar] [CrossRef]
- Gutiérrez-Soto, G.; Medina-González, G.; Treviño-Ramirez, J.; Hernández-Luna, C. Native macrofungi that produce lignin-modifyieng enzymes, cellulases, and xylanases with potential biotechnological applications. Bioresources 2015, 10, 6676–6689. [Google Scholar]
- Osono, T.; Takeda, H. Comparison of litter decomposing ability among diverse fungi in a cool temperate deciduous forest in Japan. Mycologia 2002, 94, 421–427. [Google Scholar] [CrossRef]
- Koide, K.; Osono, T.; Takeda, H. Fungal succession and decomposition of Camellia japonica leaf litter. Ecol. Res. 2005, 20, 599–609. [Google Scholar] [CrossRef]
- Bezerra, J.; Santos, M.; Svedese, V.; Lima, D.; Fernandes, M.; Paiva, L.; Souza-Motta, C. Richness of endophytic fungi isolated from Opuntia ficus-indica Mill. (Cactaceae) and preliminary screening for enzyme production. World J. Microbiol. Biotechnol. 2012, 28, 1989–1995. [Google Scholar] [CrossRef]
- Chaparro, D.; Rosas, D.; Varela, A. Aislamiento y evaluación de la actividad enzimática de hongos descomponedores de madera (Quindío, Colombia). Rev. Iberoam. Micol. 2009, 26, 238–243. [Google Scholar] [CrossRef]
- Moissenko, K.; Vasina, D.; Farukshina, K.; Savinova, O.; Glazunova, O.; Fedorova, T.; Tyashelova, T. Orchestration of the expression of the laccase multigene family in white-rot basidiomycete Trametes hirsuta 972: Evidences of transcription level subfunctionalization. Fungal Biol. 2018, 122, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Negrão, D.; Fernandes da Silva J#xFA;nior, T.; de Souza Passos, J.; Angeli Sansílogo, C.; de Almeida Minhoni, M.; Furtado, E. Biodegradation of Eucalyptus urograndis wood by fungi. Int. Biodeterior. Biodegrad. 2014, 89, 95–102. [Google Scholar]
- Liers, C.; Ullrich, R.; Steffen, K.T.; Hatakka, A.; Hofrichllter, M. Mineralization of 14C-labelled synthetic lignin and extracellular enzyme activities of the wood-colonizing ascomycetes Xylaria hypoxylon and Xylaria polymorpha. Appl. Microbiol. Biotechnol. 2006, 69, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Coseri, S. Cellulose: To depolymerize or not to? Biotechnol. Adv. 2017, 35, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, C.; Mazzarino, M.; Laos, F. Improving the quality of municipal organic waste compost. Bioresour. Thechnol. 2006, 98, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Vyas, T.K.; Dave, B.P. Effect of addition of nitrogen, phosphorus and potassium fertilizers on biodegradation of crude oil by marine bacteria. Indian J. Mar. Sci. 2010, 39, 143–150. [Google Scholar]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Merhautováa, V.; Zervakis, G. Olive mill wastewater biodegradation potential of white-rot fungi—Mode of action of fungal culture extracts and effects of ligninolytic enzymes. Bioresour. Technol. 2015, 189, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Camacho, R.; Evans, C.; Hedger, J. Production of ligninolytic enzymes by species assemblages of tropical higher fungi from Ecuador. In Tropical Mycology; Watling, R., Frankland, J.C., Ainsworth, A.M., Isac, S., Robinson, C.H., Eds.; CABI Publishing: Wallingford, UK, 2002; Volume 1, pp. 101–112. [Google Scholar]
- Vaca, M.; Izurieta, B.; Espín, N. Obtención de extractos enzimáticos con actividad celulolítica y ligninolítica a partir del hongo Pleurotus ostreatus 404 y 2171 en rastrojo de maíz. Revista Politec. 2014, 33, 2. [Google Scholar]
- Hladki, A.; Romero, A. A preliminary account of Xylaria in the Tucuman Province, Argentina, with a key to the known species from the northern provinces. Fungal Divers. 2010, 42, 79–96. [Google Scholar] [CrossRef]
- Medel, R.; Castillo, R.; Guzmán, G. Adiciones al conocimiento de Xylaria (Ascomycota, Xylariales) en México. Rev. Mex. Micol. 2010, 31, 9–18. [Google Scholar]
- Ministerio del Ambiente de Ecuador (MAE). Mapa de Cobertura y Uso de la Tierra. Available online: http://www.ambiente.gob.ec (accessed on 24 April 2017).
- Iotti, M.; Zambonelli, A. A quick and precise technique for identifying ectomycorrhizas by PCR. Mycol. Res. 2006, 110, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, N., Gelfand, D., Sninsky, J., White, J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Ruqayyah, T.; Jamal, P.; Alam, Z.; Mirghani, E. Biodegradation potential and ligninolytic enzyme of two locally isolated Panus tigrinus strain on selected agro-industrial wastes. J. Environ. Manag. 2013, 118, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds II: A rapid method for the determination of fibre and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage fiber analyses (apparatus, reagents, procedures, and some applications). In Agriculture Handbook No. 379; U.S. Agricultural Research Service: Washington, DC, USA, 1970. [Google Scholar]
- Sommer, L.; Nelson, D. Determination of total phosphorus in soils: A rapid perchloric acid digestion procedure. Soil Sci. Soc. Am. Proc. 1972, 53, 32–37. [Google Scholar] [CrossRef]
- Liers, C.; Ullrich, R.; Pecyna, M.; Schlosser, D.; Hofrichter, M. Production, purification and partial enzymatic and molecular characterization of a laccase from the wood-rotting ascomycete Xylaria polymorpha. Enzyme Microb. Tech. 2007, 41, 785–793. [Google Scholar] [CrossRef]
- Roé-Sosa, A.; Estrada, M.; Calderas., F.; Sánchez-Arévalo, F.; Manero, O.; Orta, L.; de Velasquez, M.T. Degradation and biodegradation of polyethylene whit pro-oxidant additives under compost conditions establishing relationships between physicochemical and rheological parameters. J. Appl. Polym. Sci. 2015, 42721, 1–11. [Google Scholar]
- Wei, D.; Chou, H.; Cheng, M.; Chang, S. Purification and characterization of xylanase from Xylaria regalis. Fung. Sci. 2005, 20, 53–59. [Google Scholar]
- Dong, X.Q.; Yang, J.S.H.; Zhu, N.; Wang, E.T.; Yuan, H.L. Sugarcane bagasse degradation and characterization of three white-rot fungi. Bioresour. Technol. 2013, 131, 443–451. [Google Scholar] [CrossRef]
- Mata, G.; Savoie, J. Extracelullar enzyme activities in six Lentinula edodes strains during cultivation in wheat straw. World J. Microb. Biot. 1998, 14, 513–519. [Google Scholar] [CrossRef]
- Philippoussis, A.; Diamantopoulou, P.; Papadopoulou, K.; Lakhtar, H.; Roussos, S.; Parissopoulos, G.; Papanikolaou, S. Biomass, laccase and endoglucanase production by Lentinula edodes during solid state fermentation of reed grass, bean stalks and wheat straw residues. World J. Microb. Biot. 2011, 27, 285–297. [Google Scholar] [CrossRef]
- Sadaf, A.; Khare, S.K. Production of Sporotrichum thermophile xylanase by solid state fermentation utilizing deoiled Jatropha curcas seed cake and its application in xylooligosachharide synthesis. Bioresour. Technol. 2014, 153, 126–130. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosaIicyIic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Singhania, R.; Saini, J.K.; Saini, R.; Adsul, M.; Mathur, A.; Gupta, R.; Tuli, D.K. Bioethanol production from wheat straw via enzymatic route employing Penicillium janthinellum cellulases. Bioresour. Technol. 2014, 169, 490–495. [Google Scholar] [CrossRef]
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/KF192827 (accessed on 24 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/JX256824 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/KJ767110 and KJ767104 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/AB569622 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/AB809464 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/HM992808 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/GU322460, GU991523, GU300095, GU300088, EF026123, EF026149 and GU300086 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/EU715634 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/JN198529 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/JF440974 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/nuccore/KT250967, KT250968, KT250969, KT250970, KT250971, KT250972, KT250973, KT250974, KT250975, KT250976 and KT250977 (accessed on 14 June 2016).
- Zhang, H.; Schuchardt, F.; Li, G.; Yang, J.; Yang, Q. Emission of volatile sulfur compounds during composting of municipal solid waste (MSW). Waste Manag. 2013, 33, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, Y.; Li, Y. Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion. Bioresour. Technol. 2014, 156, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Liu, G.; Chen, C.; He, Y.; Liu, X. Comparison of methane production potential, biodegradability, and kinetics of different organic substrates. Bioresour. Technol. 2013, 149, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yan, H.; Liu, Y.; Huang, Y.; Zhang, R.; Chen, C.; Liu, G. Bio-energy conversion performance, biodegradability, and kinetic analysis of different fruit residues during discontinuous anaerobic digestion. Waste Manag. 2016, 52, 295–301. [Google Scholar] [CrossRef]
- Santos Michel, R.J., Jr.; Canabarro, N.I.; Alesio, C.; Maleski, T.; Laber, T.; Sfalcin, P.; Foletto, E.; Mayer, F.D.; Kuhn, R.C.; Mazutti, M. Enzymatic saccharification and fermentation of rice processing residue for ethanol production at constant temperature. Biosyst. Eng. 2016, 142, 110–116. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas production from algal biomas: A review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Sampedro, I.; Marinari, S.; D’Annibale, A.; Grego, S.; Ocampo, J.; García-Romera, I. Organic matter evolution and partial detoxification in two-phase olive mill waste colonized by white-rot fungi. Int. Biodeterior. Biodegrad. 2007, 60, 116–125. [Google Scholar] [CrossRef]
- El-Haddad, M.; Zayed, M.; El-Sayed, G.; Hassanein, M.; El-Satar, A. Evaluation of compost, vermicompost and their teas produced from rice straw as affected by addition of different supplements. Ann. Agric. Sci. 2014, 59, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Bullerman, L. Effects of potassium sorbate on growth and aflatoxin production by Aspergillus parasiticus and Aspergillus flavus. J. Food Protect. 1983, 46, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Eze, J.M. Translocation of phosphate in mould mycelia. New Phytol. 1975, 75, 579–581. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, G.; Zhang, R.; Hu, D.; Wang, H.; Ng, T. A novel aspartic protease with HIV-1 reverse transcriptase inhibitory activity from fresh fruiting bodies of the wild mushroom Xylaria hypoxylon. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Pointing, S.; Parungao, M.; Hyde, K. Production of wood-decay enzymes, mass loss and lignin solubilization in wood by tropical Xylariaceae. Mycol. Res. 2003, 107, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Digby, A.; Gleason, F.; Mcgee, P. Some fungi in the Chytridiomycota can assimilate both inorganic and organic sources of nitrogen. Fungal Ecol. 2010, 3, 261–266. [Google Scholar] [CrossRef]
- Fogarty, W.; Kelly, C. Microbial Enzymes and Biotechnology; Fogarty, W.M., Kelly, C., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 71–472. [Google Scholar]
- Rigby, H.; Clarke, B.; Pritchard, D.; Meehan, B.; Beshah, F.; Smith, S.; Porter, N. A critical review of nitrogen mineralization in biosolids-amended soil, the associated fertilizer value for crop production and potential for emissions to the environment. Sci. Total Environ. 2016, 541, 1310–1338. [Google Scholar] [CrossRef]
- Fioretto, A.; Di Nardo, C.; Papa, S.; Fuggi, A. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 1083–1091. [Google Scholar] [CrossRef]
- Ngo, P.; Rumpel, C.; Ngo, Q.; Alexis, M.; Velásquez Vargas, G.; Mora Gil, M.; Dang, D.; Jouquet, P. Biological and chemical reactivity and phosphorus forms of buffalo manure compost, vermicompost and their misture with biochar. Bioresour. Technol. 2013, 148, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, K.A.; Suberkropp, K. Decomposition of standing litter of the freshwater emergent macrophyte Juncus effuses. Freshw. Biol. 1998, 40, 717–727. [Google Scholar] [CrossRef]
- Sujatha, S.; Bhat, R. Impacts of vermicompost and nitrogen, phosphorus, and potassium application on soil fertility status in arecanut grown on a laterite soil. Commun. Soil Sci. Plant Anal. 2012, 43, 2400–2412. [Google Scholar] [CrossRef]
- Hu, W.; Coomer, T.; Loka, D.; Oosterhuis, D.; Zhou, Z. Potassium deficiency effects the carbon-nitrogen balance in cotton leaves. Plant Physiol. Biochem. 2017, 115, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Dinis, M.J.; Bezerra, R.M.; Nunes, F.; Dias, A.; Guedes, C.; Ferreira, L.M.; Cone, J.W.; Marques, G.; Barros, A.; Rodrigues, M. Modification of wheat straw lignin by solid state fermentation with-rot fungi. Bioresour. Technol. 2009, 100, 4829–4835. [Google Scholar] [CrossRef]
- Osada, M.; Hiyoshi, N.; Sato, O.; Arai, K.; Shirai, M. Effect of sulfur on catalytic gasification of lignin in supercritical water. Energy Fuels 2007, 21, 1400–1405. [Google Scholar] [CrossRef]
- Brijwani, K.; Rigdon, A.; Vadlant, P. Fungal laccase: Production, function, and applications in food processing. Enzyme Res. 2010. [Google Scholar] [CrossRef]
- Coronel, L.M.; Joson, L.M.; Mesina, O.G. Isolation and screening of thermophilic fungi for cellulose production. Philipp. J. Sci. 1991, 120, 379–389. [Google Scholar]
- Irbe, I.; Elisashvili, V.; Asatiani, M.; Janberga, A.; Andersone, I.; Andersons, B.; Biziks, V.; Grinins, J. Lignocellulolytic activity of Coniophora puteana and Trametes versicolor in fermentation of wheat bran and decay of hydrothermally modified hardwoods. Int. Biodeterior. Biodegrad. 2014, 86, 71–78. [Google Scholar] [CrossRef]
- Sukumaran, R.; Singhania, R.; Pandey, A. Microbial cellulases—Production, applications and challenges. J. Sci. Ind. Res. India 2005, 64, 832–844. [Google Scholar]
- Arantes, V.; Saddler, J. Access to cellulose limits the efficiency of enzymatic hydrolysis: The role of amorphogenesis. Biotechnol. Biofuels 2010, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Pu, G.; Shao, C.; Cheng, S.; Cai, J.; Zhou, L.; Jía, Y.; Tian, X. Potencial of extracellular enzymes from Trametes versicolor F21a in Mycrocystis spp. degradation. Mater. Sci. Eng. C 2015, 48, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.; Vega, M.; Lienqueo, M.; Garcia, A.; Carmona, R.; Salazar, O. Cloning of novel cellulases from cellulolytic fungi: Heterologous expression of a family 5 glycoside hidrolase from Trametes versicolor in Pichia pastoris. Enzyme Microb. Tech. 2011, 49, 485–491. [Google Scholar] [CrossRef] [PubMed]




| Species Isolates | Collection Number and DNA Region | GenBank Accession No. | Country | Reference |
|---|---|---|---|---|
| Xylaria feejeensis | EGJMP22 ITS-5.8S | KF192827 | India | Jagan et al. [50] |
| Xylaria feejeensis | EGJMP30 ITS-5.8S | KF177680 | India | Jagan et al. [50] |
| Xylaria feejeensis | HMJAU 22039 ITS-5.8S | JX256824 | China | Ma et al. [51] |
| Xylaria feejeensis | A2S4-D46 ITS-5.8S | KJ767110 | Malaysia | Teh & Latiffah. [52] |
| Xylaria feejeensis | A1S3-D88 ITS-5.8S | KJ767104 | Malaysia | Teh & Latiffah. [52] |
| Xylaria feejeensis | Genes; ITS-5.8S | AB569622 | Japan | Siriwach et al. [53] |
| Xylaria feejeensis | Genes; ITS-5.8S | AB809464 | Tailandia | Srisapoomi et al. [54] |
| Xylaria feejeensis | E6912b ITS-5.8S | HM992808 | USA | Bascom-Slack et al. [55] |
| Xylaria feejeensis | 1012 ITS-5.8S | GU322460 | --- | Hsieh et al. [56] |
| Xylaria feejeensis | 860 ITS-5.8S | GU991523 | --- | Hsieh et al. [56] |
| Xylaria curta | SGLAf81 ITS-5.8S | EU715634 | --- | Soca-Chafre et al. [57] |
| Xylaria hypoxylon | 95082001 ITS-5.8S | GU300095 | --- | Hsieh et al. [56] |
| Xylaria grammica | 152 ITS-5.8S | KF312440 | --- | Jagan et al. [50] |
| Xylaria bambusicola | 162 ITS-5.8S | GU300088 | --- | Hsieh et al. [56] |
| Xylaria bambusicola | 205 ITS-5.8S | EF026123 | --- | Hsieh et al. [56] |
| Xylaria venosula | 94080508 ITS-5.8S | EF026149 | --- | Hsieh et al. [56] |
| Xylaria venosula | ITS-5.8S | JN198529 | --- | Wu et al. [58] |
| Xylaria microceras | 414 ITS-5.8S | GU300086 | --- | Hsieh et al. [56] |
| Collodiscula japonica | CJ ITS-5.8S | JF440974 | Austria | Jaklitsch & Voglmayr [59] |
| Xylaria cf. microceras | 1m_VC ITS-5.8S | KT250967 | Ecuador | Carrión-Paladines et al. [60] |
| Xylaria feejeensis | Cb_VC4 ITS-5.8S | KT250968 | Ecuador | Carrión-Paladines et al. [60] |
| Xylaria feejeensis | Cn_VC3ITS-5.8S | KT250969 | Ecuador | Carrión-Paladines et al. [60] |
| Xylaria cf. microceras | VCF1 ITS-5.8S | KT250970 | Ecuador | Carrión-Paladines et al. [60] |
| Xylaria feejeensis | VCF10 ITS-5.8S | KT250971 | Ecuador | Carrión-Paladines et al. [60] |
| Xylaria feejeensis | VCF3c ITS-5.8S | KT250972 | Ecuador | Carrión-Paladines et al. [60] |
| Xylaria feejeensis | VCF4c ITS-5.8S | KT250973 | Ecuador | Carrión-Paladines et al. [60] |
| Xylaria cf. microceras | VCF7 ITS-5.8S | KT250974 | Ecuador | Carrión-Paladines et al. [60] |
| Xylaria cf. microceras | VCF7c ITS-5.8S | KT250975 | Ecuador | Carrión-Paladines et al. [60] |
| Xylaria feejeensis | VCF9c ITS-5.8S | KT250976 | Ecuador | Carrión-Paladines et al. [60] |
| Xylaria cf. microceras | xml_ VC8 ITS-5.8S | KT250977 | Ecuador | Carrión-Paladines et al. [60] |
| Biochemical Compound | Average | Standard Deviation |
|---|---|---|
| Lignin (%) | 9.1 | 0.79 |
| Cellulose (%) | 19.5 | 0.91 |
| TC (%) | 47.3 | 0.20 |
| TN (%) | 1.5 | 0.08 |
| C/N ratio | 30.4 | 1.57 |
| TP (%) | 0.2 | 0.02 |
| TK (%) | 1.2 | 0.20 |
| S (%) | 0.0 | 0.00 |
| pH | 7.0 | 0.16 |
| Parameter | X. feejeensis | X. cf. microceras | T. versicolor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days of Incubation | Days of Incubation | Days of Incubation | ||||||||||
| 7 | 15 | 30 | 60 | 7 | 15 | 30 | 60 | 7 | 15 | 30 | 60 | |
| TC (%) | 28.4 ± 0.8 | 24.2 ± 1.0 | 20.7 ± 1.1 | 17.0 ± 0.4 | 28 ± 1.0 | 25.4 ± 1.2 | 21.7 ± 1.2 | 19.1 ± 1.1 | 26.7 ± 0.4 | 22.7 ± 0.7 | 19.6 ± 0.8 | 16.1 ± 0.7 |
| TN (%) | 1.4 ± 0.0 | 1.4 ± 0.1 | 1.5 ± 0.0 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.0 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.0 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.0 |
| C/N ratio | 20.7 ± 0.4 | 17.2 ± 1.3 | 14.1 ± 1.0 | 11.9 ± 0.7 | 21.4 ± 1.8 | 18.4 ± 0.6 | 15.0 ± 0.7 | 13.3 ± 0.9 | 21.1 ± 0.4 | 16.6 ± 1.0 | 13.8 ± 0.8 | 11.7 ± 0.5 |
| TP (%) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| TK (%) | 0.8 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.0 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.0 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.0 |
| Cellulose (%) | TC (%) | TN (%) | C/N | TP (%) | TK (%) | |
|---|---|---|---|---|---|---|
| Lignin loss (%) | 0.457 ** | 0.581 ** | −0.377 ** | 0.564 ** | −0.265 * | 0.164 |
| Cellulose (%) | 0.821 ** | −0.683 ** | 0.835 ** | −0.372 ** | 0.383 ** | |
| TC (%) | −0.666** | 0.967 ** | −0.446 ** | 0.442 ** | ||
| TN (%) | −0.827 ** | 0.550 ** | -0.636 ** | |||
| C/N | −0.516 ** | 0.571 ** | ||||
| TP (%) | −0.385 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrión-Paladines, V.; Fries, A.; Caballero, R.E.; Pérez Daniëls, P.; García-Ruiz, R. Biodegradation of Residues from the Palo Santo (Bursera graveolens) Essential Oil Extraction and Their Potential for Enzyme Production Using Native Xylaria Fungi from Southern Ecuador. Fermentation 2019, 5, 76. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation5030076
Carrión-Paladines V, Fries A, Caballero RE, Pérez Daniëls P, García-Ruiz R. Biodegradation of Residues from the Palo Santo (Bursera graveolens) Essential Oil Extraction and Their Potential for Enzyme Production Using Native Xylaria Fungi from Southern Ecuador. Fermentation. 2019; 5(3):76. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation5030076
Chicago/Turabian StyleCarrión-Paladines, Vinicio, Andreas Fries, Rosa Elena Caballero, Pablo Pérez Daniëls, and Roberto García-Ruiz. 2019. "Biodegradation of Residues from the Palo Santo (Bursera graveolens) Essential Oil Extraction and Their Potential for Enzyme Production Using Native Xylaria Fungi from Southern Ecuador" Fermentation 5, no. 3: 76. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation5030076