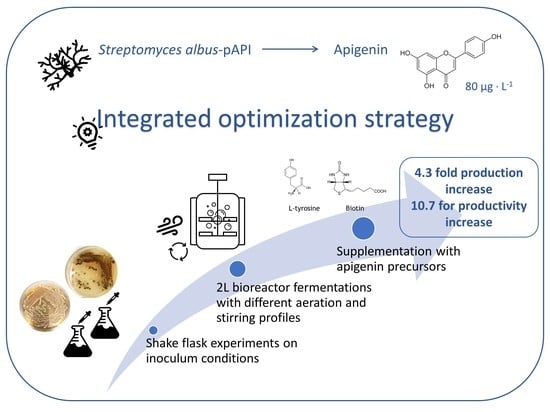
Optimization of Pre-Inoculum, Fermentation Process Parameters and Precursor Supplementation Conditions to Enhance Apigenin Production by a Recombinant Streptomyces albus Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganism and Media
2.3. Shake Flask Experiments
2.4. Batch Experiments
2.5. Analytical Methods
2.5.1. Carbon Source Determination by HPAE-PAD
2.5.2. Apigenin Extraction and Determination by UHPLC
2.5.3. L-tyrosine Determination by UHPLC
2.6. Scanning Electron Microscope Analyses
2.7. Data and Statistical Analyses
3. Results
3.1. Shake Flask Experiments
3.2. Bioreactor Experiments
3.3. Apigenin Precursor Supplementation in Shake Flask and Batch Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods; U.S. Department of Agriculture: Betsville, MD, USA, 2011.
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A review on flavonoid apigenin: Dietary Intake, ADME, antimicrobial effects, and interactions with human gut microbiota. Biomed. Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Del-Río, I.; Fernández, J.; Lombó, F. Plant Nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob Agents 2018, 52, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise (Review). Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.-M.; Ma, R.-H.; Ni, Z.-J.; Thakur, K.; Cespedes-Acuña, C.L.; Jiang, L.; Wei, Z.-J. Apigenin 7-O-glucoside promotes cell apoptosis through the PTEN/PI3K/AKT pathway and inhibits cell migration in cervical cancer HeLa cells. Food Chem. Toxicol. 2020, 146, 111843. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Park, K.-S.; Nam, H.-S.; Cho, M.-K.; Lee, S.-H. Apigenin causes necroptosis by inducing ROS accumulation, mitochondrial dysfunction, and ATP depletion in malignant mesothelioma cells. Korean J. Physiol. Pharmacol. 2020, 24, 493–502. [Google Scholar] [CrossRef]
- Korga-Plewko, A.; Michalczyk, M.; Adamczuk, G.; Humeniuk, E.; Ostrowska-Lesko, M.; Jozefczyk, A.; Iwan, M.; Wojcik, M.; Dudka, J. Apigenin and hesperidin downregulate DNA repair genes in MCF-7 breast cancer cells and augment doxorubicin toxicity. Molecules 2020, 25, 4421. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; He, W.; Xia, S.; Jiang, X.; Li, X.; Bai, J.; Li, N.; Chen, L.; Yang, B. Apigenin enhanced antitumor effect of cisplatin in lung cancer via inhibition of cancer stem cells. Nutr. Cancer 2021, 73, 1489–1497. [Google Scholar] [CrossRef]
- Woo, J.-S.; Choo, G.-S.; Yoo, E.-S.; Kim, S.-H.; Lee, J.-H.; Han, S.-H.; Kim, H.-J.; Jung, S.-H.; Park, Y.-S.; Kim, B.-S.; et al. Apigenin induces apoptosis by regulating Akt and MAPK pathways in human melanoma cell A375SM. Mol. Med. Rep. 2020, 22, 4877–4889. [Google Scholar] [CrossRef]
- Gentile, D.; Fornai, M.; Colucci, R.; Pellegrini, C.; Tirotta, E.; Benvenuti, L.; Segnani, C.; Ippolito, C.; Duranti, E.; Virdis, A.; et al. The flavonoid compound apigenin prevents colonic inflammation and motor dysfunctions associated with high fat diet-induced obesity. PLoS ONE 2018, 13, e0195502. [Google Scholar] [CrossRef]
- Ren, K.; Jiang, T.; Zhou, H.-F.; Liang, Y.; Zhao, G.-J. Apigenin retards atherogenesis by promoting ABCA1-mediated cholesterol efflux and suppressing inflammation. Cell. Physiol. Biochem. 2018, 47, 2170–2184. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Doseff, A.I. The targeted impact of flavones on obesity-induced inflammation and the potential synergistic role in cancer and the gut microbiota. Molecules 2020, 25, 2477. [Google Scholar] [CrossRef]
- Tantowi, N.A.C.A.; Mohamed, S.; Lau, S.F.; Hussin, P. Comparison of diclofenac with apigenin-glycosides rich Clinacanthus Nutans extract for amending inflammation and catabolic protease regulations in osteoporotic-osteoarthritis Rat Model. DARU J. Pharm. Sci. 2020, 28, 443–453. [Google Scholar] [CrossRef]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2018, 128, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Schobel, B.; Scott-Anne, K.; Watson, G.; Bowen, W.H.; Cury, J.A.; Rosalen, P.L.; Park, Y.K. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 2005, 84, 1016–1020. [Google Scholar] [CrossRef]
- Dong, J.; Qiu, J.; Wang, J.; Li, H.; Dai, X.; Zhang, Y.; Wang, X.; Tan, W.; Niu, X.; Deng, X.; et al. Apigenin alleviates the symptoms of Staphylococcus aureus pneumonia by inhibiting the production of alpha-hemolysin. FEMS Microbiol. Lett. 2013, 338, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-H.; Weng, B.-C.; Wu, C.-C.; Yang, S.-F.; Wu, D.-C.; Wang, Y.-C. Apigenin has anti-atrophic gastritis and anti-gastric cancer progression effects in Helicobacter pylori-infected mongolian gerbils. J. Ethnopharmacol. 2014, 151, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ma, L.; Wen, Y.; Wang, H.; Zhang, S. Studies of the in vitro antibacterial activities of several polyphenols against clinical isolates of methicillin-resistant Staphylococcus aureus. Molecules 2014, 19, 12630–12639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.-C.; Ng, L.-T.; Cheng, P.-W.; Chiang, W.; Lin, C.-C. Antiviral activities of extracts and selected pure constituents of Ocimumbasilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Shibata, C.; Ohno, M.; Otsuka, M.; Kishikawa, T.; Goto, K.; Muroyama, R.; Kato, N.; Yoshikawa, T.; Takata, A.; Koike, K. The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virology 2014, 462, 42–48. [Google Scholar] [CrossRef]
- Rameshkumar, M.R.; Indu, P.; Arunagirinathan, N.; Venkatadri, B.; El-Serehy, H.A.; Ahmad, A. Computational selection of flavonoid compounds as inhibitors against SARS-CoV-2 main protease, RNA-dependent RNA polymerase and spike proteins: A molecular docking study. Saudi J. Biol. Sci. 2021, 28, 448–458. [Google Scholar] [CrossRef]
- Trantas, E.A.; Koffas, M.A.G.; Xu, P.; Ververidis, F. When plants produce not enough or at all: Metabolic engineering of flavonoids in microbial hosts. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.K.; Alerding, A.B.; Crosby, K.C.; Bandara, A.B.; Westwood, J.H.; Winkel, B.S.J. Functional analysis of a predicted flavonol synthase gene family in Arabidopsis. Plant Physiol. 2008, 147, 1046–1061. [Google Scholar] [CrossRef] [Green Version]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Marín, L.; Gutiérrez-del-Río, I.; Yagüe, P.; Manteca, Á.; Villar, C.J.; Lombó, F. De novo biosynthesis of apigenin, luteolin, and eriodictyol in the actinomycete Streptomyces albus and production improvement by feeding and spore conditioning. Front. Microbiol. 2017, 8, 921. [Google Scholar] [CrossRef] [Green Version]
- Leonard, E.; Yan, Y.; Lim, K.H.; Koffas, M.A.G. Investigation of two distinct flavone synthases for plant-specific flavone biosynthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005, 71, 8241–8248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, E.; Chemler, J.; Lim, K.H.; Koffas, M.A.G. Expression of a soluble flavone synthase allows the biosynthesis of phytoestrogen derivatives in Escherichia coli. Appl. Microbiol. Biotechnol. 2006, 70, 85–91. [Google Scholar] [CrossRef]
- Park, S.R. Biosynthesis of plant-specific flavones and flavonols in Streptomyces venezuelae. J. Microbiol. Biotechnol. 2010, 20, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Miyahisa, I.; Funa, N.; Ohnishi, Y.; Martens, S.; Moriguchi, T.; Horinouchi, S. Combinatorial biosynthesis of flavones and flavonols in Escherichia coli. Appl. Microbiol. Biotechnol. 2006, 71, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, B.G.; Kim, M.; Ahn, J.-H. Biosynthesis of two flavones, apigenin and genkwanin, in Escherichia coli. J. Microbiol. Biotechnol. 2015, 25, 1442–1448. [Google Scholar] [CrossRef]
- Thuan, N.H.; Chaudhary, A.K.; Van Cuong, D.; Cuong, N.X. Engineering co-culture system for production of apigetrin in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2018, 45, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Song, M.C.; Ban, Y.H.; Jun, S.Y.; Kwon, A.S.; Lee, J.Y.; Yoon, Y.J. High-yield production of multiple O-methylated phenylpropanoids by the engineered Escherichia coli–Streptomyces cocultivation system. Microb. Cell Fact. 2019, 18, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbuto Ferraiuolo, S.; Cammarota, M.; Schiraldi, C.; Restaino, O.F. Streptomycetes as platform for biotechnological production processes of drugs. Appl. Microbiol. Biotechnol. 2021, 105, 551–568. [Google Scholar] [CrossRef]
- Olmos, E.; Mehmood, N.; Haj Husein, L.; Goergen, J.-L.; Fick, M.; Delaunay, S. Effects of Bioreactor Hydrodynamics on the Physiology of Streptomyces. Bioprocess Biosyst. Eng. 2013, 36, 259–272. [Google Scholar] [CrossRef]
- Sanchez, J. New insights in Streptomyces fermentations. Ferment. Technol. 2012, 1. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.; Olek, A.; Walter, J.; Sanchez, J. Analysis of DNA methylation processes related to the inhibition of DNA synthesis by 5-azacytidine in Streptomyces antibioticus ETH 7451. Biol. Chem. 1998, 379, 559–562. [Google Scholar] [PubMed]
- Restaino, O.F.; Marseglia, M.; De Castro, C.; Diana, P.; Forni, P.; Parrilli, M.; De Rosa, M.; Schiraldi, C. Biotechnological transformation of hydrocortisone to 16α-hydroxy hydrocortisone by Streptomyces roseochromogenes. Appl. Microbiol. Biotechnol. 2014, 98, 1291–1299. [Google Scholar] [CrossRef]
- Restaino, O.F.; Marseglia, M.; Diana, P.; Borzacchiello, M.G.; Finamore, R.; Vitiello, M.; D’Agostino, A.; De Rosa, M.; Schiraldi, C. Advances in the 16α-hydroxy transformation of hydrocortisone by Streptomyces roseochromogenes. Process Biochem. 2016, 51, 1–8. [Google Scholar] [CrossRef]
- Restaino, O.F.; di Lauro, I.; Cimini, D.; Carlino, E.; De Rosa, M.; Schiraldi, C. Monosaccharide precursors for boosting chondroitin-like capsular polysaccharide production. Appl. Microbiol. Biotechnol. 2013, 97, 1699–1709. [Google Scholar] [CrossRef]
- Restaino, O.F.; di Lauro, I.; Di Nuzzo, R.; De Rosa, M.; Schiraldi, C. New insight into chondroitin and heparosan-like capsular polysaccharide synthesis by profiling of the nucleotide sugar precursors. Biosci. Rep. 2017, 37, BSR20160548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, X.; Li, Y.; Tang, A.; Ren, Y. Simultaneous determination of phenylalanine and tyrosine in peripheral capillary blood by HPLC with ultraviolet detection. Clin. Biochem. 2013, 46, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Barbuto Ferraiuolo, S.; Perna, A.; Cammarota, M.; Borzacchiello, M.G.; Fiorentino, A.; Schiraldi, C. Biotechnological transformation of hydrocortisone into 16α-hydroxyprednisolone by coupling Arthrobacter simplex and Streptomyces roseochromogenes. Molecules 2020, 25, 4912. [Google Scholar] [CrossRef]
- Myronovskyi, M.; Tokovenko, B.; Brötz, E.; Rückert, C.; Kalinowski, J.; Luzhetskyy, A. Genome rearrangements of Streptomyces albus J1074 lead to the carotenoid gene cluster activation. Appl. Microbiol. Biotechnol. 2014, 98, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Zaburannyi, N.; Rabyk, M.; Ostash, B.; Fedorenko, V.; Luzhetskyy, A. Insights into naturally minimised Streptomyces albus J1074 Genome. BMC Genom. 2014, 15, 97. [Google Scholar] [CrossRef] [Green Version]
- Baltz, R.H. Streptomyces and Saccharopolyspora hosts for heterologous expression of secondary metabolite gene clusters. J. Ind. Microbiol. Biotechnol. 2010, 37, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Yepes-García, J.; Caicedo-Montoya, C.; Pinilla, L.; Toro, L.F.; Ríos-Estepa, R. Morphological differentiation of Streptomyces clavuligerus exposed to diverse environmental conditions and its relationship with clavulanic acid biosynthesis. Processes 2020, 8, 1038. [Google Scholar] [CrossRef]
- Pinto, L.S.; Vieira, L.M.; Pons, M.N.; Fonseca, M.M.R.; Menezes, J.C. Morphology and viability analysis of Streptomyces clavuligerus in industrial cultivation systems. Bioprocess Biosyst. Eng. 2004, 26, 177–184. [Google Scholar] [CrossRef]
- Manteca, A.; Claessen, D.; Lopez-Iglesias, C.; Sanchez, J. Aerial hyphae in surface cultures of Streptomyces lividans and Streptomyces coelicolor originate from viable segments surviving an early programmed cell death event. FEMS Microbiol. Lett. 2007, 274, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manteca, A.; Alvarez, R.; Salazar, N.; Yagüe, P.; Sanchez, J. Mycelium differentiation and antibiotic production in submerged cultures of Streptomyces coelicolor. Appl. Environ. Microbiol. 2008, 74, 3877–3886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, A.A.; Vieira, L.M.; Menezes, J.C. Effects of preculture variability on clavulanic acid fermentation. Biotechnol. Bioeng. 2001, 72, 628–633. [Google Scholar] [CrossRef]
- López-García, M.T.; Álvarez, J.R. Cell Immobilization of Streptomyces coelicolor: Effect on differentiation and actinorhodin production. Int. Microbiol. 2014, 75–80. [Google Scholar] [CrossRef]
- Rosa, J.C.; Neto, A.B.; Hokka, C.O.; Badino, A.C. Influence of dissolved oxygen and shear conditions on clavulanic acid production by Streptomyces clavuligerus. Bioprocess Biosyst. Eng. 2005, 27, 99–104. [Google Scholar] [CrossRef]
- Potumarthi, R.; Ch, S.; Jetty, A. Alkaline protease production by submerged fermentation in stirred tank reactor using Bacillus licheniformis NCIM-2042: Effect of aeration and agitation regimes. Biochem. Eng. J. 2007, 34, 185–192. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, H.J.; Xu, C.P.; Choi, J.W.; Yun, J.W. Effect of aeration and agitation on the production of mycelial biomass and exopolysaccharides in an enthomopathogenic fungus Paecilomycessinclairii. Lett Appl. Microbiol. 2003, 36, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Radchenkova, N.; Vassilev, S.; Martinov, M.; Kuncheva, M.; Panchev, I.; Vlaev, S.; Kambourova, M. Optimization of the aeration and agitation speed of Aeribacilluspalidus 418 exopolysaccharide production and the emulsifying properties of the product. Process Biochem. 2014, 49, 576–582. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, L.-R.; He, H.-W.; Sang, B.; Yu, D.-L.; Feng, J.-T.; Zhang, X. Effects of agitation, aeration and temperature on production of a novel glycoprotein GP-1 by Streptomyces kanasenisi ZX01 and scale-up based on volumetric oxygen transfer coefficient. Molecules 2018, 23, 125. [Google Scholar] [CrossRef] [Green Version]
- Hofeditz, T.; Unsin, C.; Wiese, J.; Imhoff, J.; Wohlleben, W.; Grond, S.; Weber, T. Lysoquinone-TH1, a new polyphenolic tridecaketide produced by expressing the lysolipin minimal PKS II in Streptomyces albus. Antibiotics 2018, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuzawa, S.; Mirsiaghi, M.; Jocic, R.; Fujii, T.; Masson, F.; Benites, V.T.; Baidoo, E.E.K.; Sundstrom, E.; Tanjore, D.; Pray, T.R.; et al. Short-chain ketone production by engineered polyketide synthases in Streptomyces albus. Nat. Commun. 2018, 9, 4569. [Google Scholar] [CrossRef] [PubMed]






| Operating Process Parameters | Batch A | Batch B | Batch C | Batch D |
|---|---|---|---|---|
| Stirring (rpm) | 300–800 | 300–600 | 300–600 | 300–600 |
| Aeration (L·h−1) | 78–162 | 102–120 | 102–120 | 102–120 |
| pO2 Set Point (%DO) | 40 | 30 | 30 | 30 |
| Starting Inoculum (% v/v) | 5 | 5 | 10 | 10 |
| L-tyrosine Supplementation | - | - | - | 1.5 mM |
| Batch A | Batch B | Batch C | Batch D | |
|---|---|---|---|---|
| Maximum biomass (gcdw·L−1) | 10.0 ± 0.3 | 10.4 ± 0.4 | 11.1 ± 0.2 | 10.0 ± 0.1 |
| Maximum apigenin concentration (µg·L−1) | 168.9 ± 6.8 | 160.4 ± 5.2 | 184.8 ± 4.0 | 343.3 ± 3.0 |
| Maximum productivity (µg·L−1·h−1) | 1.8 ± 0.1 | 3.3 ± 0.1 | 2.6 ± 0.1 | 7.1 ± 0.1 |
| Yapig/X (µg·g−1cdw) | 17.3 ± 0.1 | 15.4 ± 0.1 | 16.7 ± 0.1 | 34.3 ± 0.1 |
| Yapig/S (µg·g−1totalc) | 3.8 ± 0.1 | 4.6 ± 0.1 | 3.2 ± 0.1 | 5.9 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbuto Ferraiuolo, S.; Restaino, O.F.; Gutiérrez-del-Río, I.; Ventriglia, R.; Cammarota, M.; Villar, C.J.; Lombó, F.; Schiraldi, C. Optimization of Pre-Inoculum, Fermentation Process Parameters and Precursor Supplementation Conditions to Enhance Apigenin Production by a Recombinant Streptomyces albus Strain. Fermentation 2021, 7, 161. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation7030161
Barbuto Ferraiuolo S, Restaino OF, Gutiérrez-del-Río I, Ventriglia R, Cammarota M, Villar CJ, Lombó F, Schiraldi C. Optimization of Pre-Inoculum, Fermentation Process Parameters and Precursor Supplementation Conditions to Enhance Apigenin Production by a Recombinant Streptomyces albus Strain. Fermentation. 2021; 7(3):161. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation7030161
Chicago/Turabian StyleBarbuto Ferraiuolo, Simona, Odile Francesca Restaino, Ignacio Gutiérrez-del-Río, Riccardo Ventriglia, Marcella Cammarota, Claudio J. Villar, Felipe Lombó, and Chiara Schiraldi. 2021. "Optimization of Pre-Inoculum, Fermentation Process Parameters and Precursor Supplementation Conditions to Enhance Apigenin Production by a Recombinant Streptomyces albus Strain" Fermentation 7, no. 3: 161. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation7030161