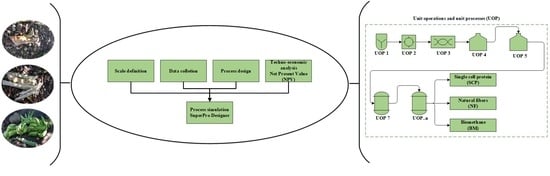
3.1.2. Process Design and Simulation
The conceptual design of the studied processes was carried out from the analysis of the 343 documents obtained from the databases. These diagrams represent the basic structure of the different unit operations and unit processes intended for the conversion of the residues (pseudostems and RUPFs) into products. The conceptual design diagrams for single-cell protein production from plantain pseudostems and RUPFs (
Figure 1), NF production from pseudostems (
Figure 2), and BM production from pseudostems (
Figure 3) are briefly described below.
The process proposed for SCP production from pseudostems (see
Figure 1a) is described as follows. For washing (E-01), the plantain residues are immersed in water (3.5 m
3 per ton) [
59] at 25 °C in tanks with agitation for 12 min to remove other waste (soil and fines). For grinding (E-02), the residues are reduced in size down to particles between 1 and 2 mm. During the first pretreatment (E-03), the residues are mixed with sulfuric acid (2.2%
v/
v) [
60]. The reaction is kept at 25 °C for 12 h with continuous stirring. In the second pretreatment (E-04), the mixture is treated by steam explosion at 177 °C for 5 min [
60]. Then, the mixture is pressed (E-05) to extract the aqueous solution (90%) enriched with monosaccharides (mainly glucose and xylose).
For saccharification (E-06), water is added to the mixture and the pH is adjusted to 5.5 [
61]. Cellulases and hemicellulases are added (6% and 2%
w/
v, respectively). The reacting mixture is continuously stirred at 45 °C for 24 h [
61]. For filtration (E-07), the solids are removed through an 80-mesh filter. During the ion exchange (E-09 and E-10), the sugar mixture (E-08) is detoxified using H
+ and OH
-/Cl
- resins [
62,
63,
64]. For sterilization (E-11), a solution containing 5 g/L ammonium sulfate is added to the mixture [
65], which is heated at 121 °C for 15 min [
15].
For fermentation (E-12), the sterilized culture medium is stored in reactors, and 10%
C. utilis inoculum is added [
15].
C. utilis was considered for fermentation due to its ability to assimilate different carbon sources, such as hexoses (e.g., glucose) and pentoses (e.g., xylose) [
66,
67]. In addition, this yeast is one of the most used for the production of SCP [
10] and it is generally recognized as safe (GRAS) [
68] for use in human food and animal feed. Then, the fermentation is kept under aerobic conditions at 30 °C and pH 4.5 for 24 h [
69]. For the reduction of nucleic acids in yeast (E-13), the culture broth is mixed with concentrated sulfuric acid (12 mL/L); this procedure is needed in order to prevent the RNA from generating uric acid in the SCP consumer. Next, the acid-treated broth is heated at 80 °C for 30 min [
70]. Afterwards, the broth is mixed with a solution of saturated sodium hydroxide up to pH 8.7 and the reaction is maintained for 10 min. For centrifugation (E-14), water is added to the resulting liquid stream continuously (at a rate of 1:1) and yeasts are separated by a disk-stack centrifuge [
70]. Finally, the yeasts are dried to 150 °C in a spray dryer (E-15) [
71], with a final moisture between 3% and 5% [
72]. SCP is packed (E-16) in high-density polyethylene (HDPE) bags to obtain 25-kg packaged product units.
The process proposed in
Figure 1b for SCP production from RUPFs is described as follows. For washing (E-1.1), the procedure briefly described above for unit E-01 is applied. For peeling (E-1.2), the peel is separated mechanically from pulp at a 150 kg/h flowrate. During grinding (E-1.3), the pulp is blended at 30,000 rpm for 10 min. In this step, 5% (
w/
v) ascorbic acid solution is added to the mixture to prevent enzyme damage [
73]; then, total solids (TSs) are diluted up to 30% [
74]. Next, 6 ppm calcium ions (as CaCl
2) per kilogram of mixture are added [
75]. The mixture pH is adjusted to 5.5, and 0.15% (
w/
v) thermostable
Bacillus licheniformis α-amylase liquid preparation is added [
75]. For liquefaction (E-1.5), steam at 105 °C is mixed directly and continuously (jet cooking) (E-1.4) with the mixture [
74]. The mixture temperature is adjusted up to 95 °C and kept under continuous stirring at 100 rpm for 3 h [
75]. For saccharification (E-1.7), the pH and temperature of the mixture are adjusted to 4.5 and 60 °C (E-1.6), respectively, and 0.15 % (
v/
v) amyloglucosidase is added [
76]. The hydrolysis is kept under continuous stirring in the reactors for 6 h. At the end of this step, the dextrose equivalent (DE) of the mixture must be greater than 80% [
76]. Next, the procedures occurring in units E-11 to E-16 are applied (see
Figure 1a).
The process proposed to produce natural fibers (
Figure 2) starts in the E-01 unit, where the leaf sheaths and floral stalk making up the pseudostems are mechanically separated. Then, the leaf sheaths are subjected to decortication (crus and bruise) using equipment with two cylinders (E-02) to extract the fibers [
18]. Next, the fibers are washed (E-03) (water to fiber ratio of 2:1) to remove pigments and other compounds (part of fats, proteins, minerals, cellulose, hemicellulose, lignin, etc.). Then, the fibers are treated with 6% (
w/
v) sodium hydroxide (ratio of 2:1) (E-04). The reaction is kept in a tank with continuous stirring at 60 °C for 2 h [
77,
78]. During washing (E-05), the fibers are submerged in water (ratio of 2:1) at room temperature [
18]. Finally, the fibers are dried (E-06) (maximum moisture of 8%) in a convection oven with air recirculation at 80 °C for 4 h [
77].
The process proposed for BM production from the pseudostems (see
Figure 3) starts with the grinding operation (E-01), where the pseudostems are reduced in size down to particles between 1 and 2 mm. Then, the particles are pretreated (E-02) by steam explosion at 200 °C for 76 min [
79]. The resulting mixture is dissolved with water down to 10–15% TS [
80]. During the anaerobic digestion (E-03), a solution containing 5 g/L ammonium sulfate is added to the mixture as a nitrogen source for the microorganisms. The process is performed under slow continuous stirring at 40 °C for 30 days [
81]. For biogas cleaning, the water from gas is condensed and separated through a decanter (unit E-04) [
81]. For desulphurization (E-06), the gas is compressed at 20 bar (E-05), then the gas is sent to an absorption column (scrubber), where it is contacted with water mist to extract the carbon dioxide and hydrogen sulfide [
81].
After building and describing the conceptual design (
Figure 1,
Figure 2 and
Figure 3), the first basic design of each one of the proposed processes was defined. The basic design of a process comprises a detailed analysis of the different unit operations and unit processes to define the process flowsheet, and information gathered from different sources used to simulate alternative operation modes [
82,
83]. In this way, the basic design includes the preparation of the process flowsheets, equipment list, corresponding mass and energy balances, and process scheduling (particularly important for batch processes). For this task, the SuperPro Designer simulator was employed in this work. In general, the basic design comprises all the documentation required to define the platform, production facility, and structure configurations and dimensions in satisfactory detail to allow the start of the detailed design (detailed engineering phase) [
84].
The definition of the process flowsheets for the production of SCP, NFs, and BM started with the selection of the time regime of the whole process (batch vs. continuous). For this, the selected regime was batchwise due to the multiple sub-operations in the equipment, as discussed in previous works [
8,
34], and the technical impossibility of performing some processes in a continuous mode.
The following step to accomplish the basic design started from the conceptual design diagrams already built for the proposed processes. The analysis of these diagrams made it possible to identify which material streams are needed to connect the different unit procedures (e.g., through pumps, screws, etc.). The definition of these needs may affect the mass or volumetric flowrates of the different process streams, increasing the number of equipment units and affecting the techno-economic indicators. Therefore, for the process of SCP production from pseudostems, the equipment units for transferring solids (E-05, E-08, and E-13) and liquids (E-18, E-25, E-28, E-30, and E-35) and for balance storage (E-17 and E-34) were defined and included in the process flowsheet (see
Figure 4). For SCP production from RUPFs, the equipment units for transferring liquids (E-09, E-12, E-15, E-21, E-24, E-26, and E-31) and for balance storage (E-08, and E-30) were included as well (
Figure 5). For NF production, some units for transferring solids (E-03, E-10, and E-13) were added (
Figure 6). Finally, for BM production, equipment units to transfer solids (E-02, E-04, and E-15) and liquids (E-06 and E-24), and for balance storage (E-05) were defined and added (
Figure 7). The inclusion of these unit procedures allowed an assessment of their impact on the techno-economic index.
The basic design continued with the analysis of the input-output structure, according to Douglas’ terminology [
85]. The need to purify the feed streams before the transformation step (E-01 in
Figure 1) made it possible to identify water recovery possibilities as an improvement of the design as discussed in previous works [
8,
34]. In this sense, for the processes of SCP production (
Figure 4 and
Figure 5), a washing step (E–01) with a water recovery system (E-02) that separates the solids (soil and fines) and adjusts the flowrate with fresh water (E-03) was included. This variant made it possible to recover up to 90% of the water used in the washing step. For NF and BM production, there is no need to purify streams due to the nature of the processes, which do not require strict cleaning.
The preparation and definition of the process flowsheet also contemplated the analysis of the reaction system. The main transformations involved in the processes for producing SCP (aerobic cultivation) and BM (anaerobic digestion) are shown in
Table 6. The reaction system corresponds to the recycle structure according to Douglas’ terminology. In this sense, a recycle structure in the process flowsheet for SCP production from pseudostems (
Figure 4) was included in the unit procedures E-24 to E-26. For SCP production from RUPFs, the recycle structure was added in the unit procedures E-20 to E-22 (
Figure 5). The recycle structure in
Figure 4 is described as follows: 10% of the sterilized mixture (E-23) is sent to the seed reactors (E-24), i.e., 10% of the
C. utilis inoculum [
15]. Fermentation is kept under aerobic conditions at 30 °C and pH 4.5 for 18 h [
69]. At the end of this step, 30% of the volume of broth in the seed reactors is mixed in the main reactors (E-27) with 90% sterilized medium (E-23). Seventy percent of the broth volume (E-26) is recirculated to the seed reactors [
69]. The recycle structure in
Figure 5 was carried out in a similar way as in
Figure 4. On the other hand, the process for NF production has no recycle structures (
Figure 6). For the process of BM production (
Figure 7), the recycle structure was added in the unit procedures E-10 to E-16. This system is described as follows: 30% of the sludge (E-16) is sent to the digesters for stabilization (E-17) and 70% is recirculated to the anaerobic digestion bioreactors (E-12 and E-13) [
86], where it is mixed with fresh waste mixture (E-10 and E-11). The recycle structure in the fermentation or anaerobic digestion steps is paramount in the design of this type of biotechnological process since recirculation provides stability and improved performance by increasing the biomass concentration and reducing the conditioning time of the cultures in these steps [
87].
According to Douglas’ methodology, the separation system is the next step during process design. In this regard, the separation of
C. utilis from the broth was carried out through a disk-stack centrifuge and rotary vacuum filter (E–32 and E–33 in
Figure 4, and E–28 and E–29 in
Figure 5). Moreover, in this step, other separation schemes were analyzed as well. For instance, the chemical pretreatment (E-07 in
Figure 4), a press system for solids (E-08) with acid solution recovery (E-09 and E-10), and flowrate adjustment (E-11) were included. This system allows the recovery of up to 90% of the aqueous solution. For the washing step (E-03 and E-10 in
Figure 6), a recovery system of aqueous solution with solids separation (E-04 and E-11) and flowrate adjustment (E-05 and E-12) was also included. It is worth highlighting from this process the recovery of the aqueous solution (water and sodium hydroxide mixture) in the unit procedure E-07 and the inclusion of a hot air recovery step (E-15) that allows the recovery of up to 90% of air. This configuration provokes a temperature increase inside the fibers, decreasing the energy requirement during the subsequent drying. For the BM process, a water recovery system was implemented [
88,
89]. This system consists of a pump (E-24) that sends water under pressure from the desulphurization step (E-23) to the degasification step (E-27), where the water is mixed with air (E-26) to extract hydrogen sulfide and carbon dioxide. Then, the water is recovered (90%) (E-25), and the temperature (E-22) and flowrate (E-21) of this stream are adjusted.
Table 6.
Reactions involved during the simulation of the process flowsheets for SCP and biomethane production.
Table 6.
Reactions involved during the simulation of the process flowsheets for SCP and biomethane production.
| Process | Steps | Reactions | Comments |
|---|
| SCP | Steam explosion (E-12) and saccharification (E-15) in Figure 4Liquefaction (E-11) and saccharification (E-14) in Figure 5 | From starch/cellulose to glucose | Extent of the hydrolysis reaction of starch to glucose in the steam explosion step was assumed as 100%. Cellulose and hemicellulose hydrolysis was assumed as 19.4% and 90%, respectively [60]. Yield of reducing sugars (mainly glucose and xylose) in saccharification was assumed as 82% [90]. Reaction extent from starch to dextrin was 84.54 %, from dextrin to maltose was 63.27 %, and from dextrin to glucose was 36.72 % [8]. Reaction extent in saccharification was assumed as 80% [76]. |
| (5) |
| (6) |
| From hemicellulose to xylose |
| (7) |
| (8) |
| Fermentation (E-24 and E-27) in Figure 4 and (E-20 and E-23) in Figure 5 | | (9) | YXS values were 0.55 g cells/g glucose [38] and 0.45 g cells/g xylose [91]. |
| (10) |
| BM | Steam explosion (E-03) | From starch and cellulose to glucose | Cellulose and hemicellulose hydrolysis was 19.4 % and 90 %, respectively [60]. |
| (11) |
| From hemicellulose to xylose |
| (12) |
| Anaerobic digestion (E-12, E-13 and E-17) | Carbohydrate hydrolysis |
| (13) |
| (14) |
| (15) |
| (16) |
| (17) |
| (18) |
| (19) |
| Lipid hydrolysis: |
| (20) |
| (21) |
| (22) |
| (23) |
| Reactions (15), (16), and (19) are subsequently applied. |
| Protein hydrolysis: |
| (24) |
| (25) |
| Reactions (15), (16), and (19) are subsequently applied. |
Process simulation of the flowsheets performed as part of the basic design of the production of SCP, NFs, and BM allowed the calculation of the global mass balances and the process scheduling of each analyzed flowsheet at a large scale. The results obtained from the simulations are shown in
Table 7.
The simulation results indicate that the process for NF production had the highest number of batches per year (346). This process had a recipe cycle time equal to the scheduled working time on each equipment (24 h). The results suggest that the productivity of the process was linked to the semi-continuous work of the equipment. This outcome can be verified from
Figure 8,
Figure 9,
Figure 10, and
Figure 11, where the equipment occupancy charts are presented. These charts show that some equipment units were scheduled to operate in parallel without waiting for the previous equipment to finish its operation. For example, in batch 1 (
Figure 8), it is identified that all the equipment units are scheduled to start at the same time. This schedule enables the material flow entering the equipment to be processed in a semi-continuous mode to increase the amount of waste processed per unit of time.
On the other hand, the process for BM production had the lowest number of batches per year (11). This result is explained by the cycle time of this process, which reaches 30 days (720 h), especially the step of anaerobic digestion (E-12, E-13, and E-17 in
Figure 11). These equipment units represent the bottleneck of the process (recipe cycle time). The bottlenecks of the processes for the production of SCP (E-27 in
Figure 8 and E-23 in
Figure 9) and NFs (E-14 in
Figure 10) were significantly lower.
The results presented in
Table 6 also show that the processes demanding the highest amounts of bulk materials correspond to the production of SCP from pseudostems and natural fibers. This result is related to the batch size and the number of batches processed per year. The potential environmental impacts that the SCP and NF processes can have, are linked to the high energy and water consumption. The SCP production from RUPFs had a high consumption of air (24.83%) and water (46.19%). The air and water consumption in the NF process was 14.87% and 10.26%, respectively. The low water consumption in the NF process is explained by the inclusion of the water recovery system during the fiber washing.