Fermentation of Clementine Juice with Lactobacillus salivarius spp. salivarius CECT 4063: Effect of Trehalose Addition and High-Pressure Homogenization on Antioxidant Properties, Mucin Adhesion, and Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Microbial Strain
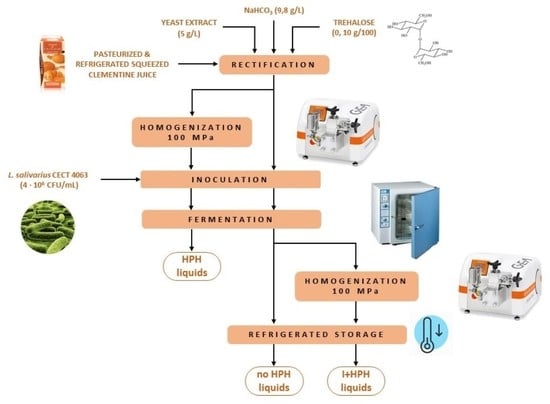
2.2. Juice Conditioning, Fermentation, and Storage
2.3. Microbial Counts
2.4. pH and Brix
2.5. Antioxidant Properties
2.5.1. Total Phenolic Content (TPC)
2.5.2. Total Flavonoid Content (TFC)
2.5.3. Antioxidant (AO) Activity
2.5.4. Quantification of Individual Flavonoids by HPLC
2.6. Adhesion Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fermentation of Clementine Juice by Lactobacillus salivarius spp. salivarius CECT 4063 as Affected by Trehalose Addition and/or Juice High-Pressure Homogenization
3.2. Adhesion to Intestinal Epithelium after Fermentation as Affected by Trehalose Addition and/or Juice Homogenization
3.3. Storage of Fermented Clementine Juice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, E.M.F.; Ramos, A.M.; Vanzela, E.S.L.; Stringheta, P.C.; de Oliveira Pinto, C.L.; Martins, J.M. Products of vegetable origin: A new alternative for the consumption of probiotic bacteria. Food Res. Int. 2013, 51, 764–770. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Working Group Report; Food and Agricultural Organization of the United Nations and World Health Organization. 2002. Available online: https://4cau4jsaler1zglkq3wnmje1-wpengine.netdna-ssl.com/wp-content/uploads/2019/04/probiotic_guidelines.pdf (accessed on 2 August 2022).
- Collado, M.C.; Lamparra, J.; Frias, R.; Lim, A.; Tan, H.M. Role of Probiotics in Health and Diseases. In Handbook of Probiotics and Prebiotics, 2nd ed.; Lee, Y., Salminen, S., Eds.; Wiley: Hoboken, NJ, USA, 2008; pp. 257–375. [Google Scholar] [CrossRef]
- FAO/WHO. Próbioticos en los Alimentos. Propiedades Saludables y Nutricionales y Directrices para la Evaluación. Estudio FAO alimentación y nutrición nº 85. 2006. Available online: https://www.fao.org/3/a0512s/a0512s.pdf (accessed on 2 August 2022).
- Panpetch, W.; Spinler, J.K.; Versalovic, J.; Tumwasorn, S. Characterization of Lactobacillus salivarius strains B37 and B60 capable of inhibiting IL-8 production in Helicobacter pylori-stimulated gastric epithelial cells. BMC Microbiol. 2016, 16, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, P.S.; Tsai, Y.C.; Chen, Y.C.; Teh, S.F.; Ou, C.M.; King, V.A.E. Eradication of Helicobacter pylori Infection by the Probiotic Strains Lactobacillus johnsonii MH-68 and L. salivarius ssp. salicinius AP-32. Helicobacter 2012, 17, 466–477. [Google Scholar] [CrossRef]
- Barrera, C.; Burca-Busaga, C.; Betoret, E.; García-Hernández, J.; Hernández, M.; Betoret, N. Improving antioxidant properties and probiotic effect of clementine juice inoculated with Lactobacillus salivarius spp. salivarius (CECT 4063) by trehalose addition and/or sublethal homogenization. Int. J. Food Sci. Technol. 2019, 54, 2109–2122. [Google Scholar] [CrossRef]
- Gao, B.; Wang, J.; Wang, Y.; Xu, Z.; Li, B.; Meng, X.; Sun, X.; Zhu, J. Influence of fermentation by lactic acid bacteria and in vitro digestion on the biotransformations of blueberry juice phenolics. Food Control 2022, 133, 108603. [Google Scholar] [CrossRef]
- Rui, X.; Wang, M.; Zhang, Y.; Chen, X.; Li, L.; Liu, Y.; Dong, M. Optimization of soy solid-state fermentation with selected lactic acid bacteria and the effect on the anti-nutritional components. J. Food Process. Preserv. 2016, 41, e13290. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Arilla, A.; Bennár, M.; Barrera, C.; Codoñer, P.; Fito, P. No invasive methodology to produce a probiotic low humid apple snack with potential effect against Helicobacter pylori. J. Food Eng. 2012, 110, 289–293. [Google Scholar] [CrossRef]
- Ohtake, S.; Wang, Y.J. Trehalose: Current use and future applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef]
- Mascorro, J.; Avonce, N.; Iturriaga, G. Biotecnología de la trehalosa en las plantas. Rev. Chapingo Ser. Hortic. 2005, 11, 193–202. [Google Scholar]
- Betoret, E.; Sentandreu, E.; Betoret, N.; Fito, P. Homogenization pressures applied to citrus juice manufacturing. Functional properties and application. J. Food Eng. 2012, 111, 28–33. [Google Scholar] [CrossRef]
- Betoret, E.; Calabuig-Jiménez, L.; Patrignani, F.; Lanciotti, R.; Dalla Rosa, M. Effect of high pressure processing and trehalose addition on functional properties of mandarin juice enriched with probiotic microorganisms. LWT-Food Sci. Technol. 2017, 85, 418–422. [Google Scholar] [CrossRef]
- Tabanelli, G.; Burns, P.; Patrignani, F.; Gardini, F.; Lanciotti, R.; Reinheimer, J.; Vinderola, G. Effect of a non-lethal High Pressure Homogenization treatment on the in vivo response of probiotic lactobacilli. Food Microbiol. 2012, 32, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Luximon-Ramma, A.; Bahorun, T.; Crozier, A.; Zbarsky, V.; Datla, K.P.; Dexter, D.T.; Aruoma, O.I. Characterization of the antioxidant functions of flavonoids and proanthocyanidins in Mauritian black teas. Food Res. Int. 2005, 38, 357–367. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Cano, A.; Medina, A.; Bermejo, A. Bioactive compounds in different citrus varieties. Discrimination among cultivars. J. Food Compost. Anal. 2008, 21, 377–381. [Google Scholar] [CrossRef]
- Izquierdo, E.; Medina, M.; Ennahar, S.; Marchioni, E.; Sanz, Y. Resistance to Simulated Gastrointestinal Conditions and Adhesion to Mucus as Probiotic Criteria for Bifidobacterium longum Strains. Curr. Microbiol. 2008, 56, 613–618. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Carbonell, J.V.; Fito, P. Effects of pressure homogenization on particle size and the functional properties of citrus juices. J. Food Eng. 2009, 92, 18–23. [Google Scholar] [CrossRef]
- Shpigelman, A.; Kyomugasho, C.; Christiaens, S.; Van Loey, A.M.; Hendrickx, M.E. The effect of high pressure homogenization on pectin: Importance of pectin source and pH. Food Hydrocoll. 2015, 43, 189–198. [Google Scholar] [CrossRef]
- Burns, P.; Patrignani, F.; Serrazanetti, D.; Vinderola, G.C.; Reinheimer, J.A.; Lanciotti, R.; Guerzoni, M.E. Probiotic Crescenza Cheese Containing Lactobacillus casei and Lactobacillus acidophilus Manufactured with High-Pressure Homogenized Milk. J. Dairy Sci. 2008, 91, 500–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velázquez-Estrada, R.M.; Hernández-Herrero, M.M.; Rüfer, C.E.; Guamis-López, B.; Roig-Sagués, A.X. Influence of ultra-high pressure homogenization processing on bioactive compounds and antioxidant activity of orange juice. Innov. Food Sci. Emerg. Technol. 2013, 18, 89–94. [Google Scholar] [CrossRef]
- Rad, A.H.; Torab, R.; Mortazavian, A.M.; Mehrabany, E.V.; Mehrabany, L.V. Can probiotics prevent or improve common cold and influenza? Nutrition 2013, 29, 805–806. [Google Scholar] [CrossRef]
- Patel, A.R. Probiotic fruit and vegetable juices- recent advances and future perspective. Int. Food Res. J. 2017, 24, 1850–1857. [Google Scholar]
- Burca-Busaga, C.; Betoret, N.; Seguí, L.; Betoret, E.; Barrera, C. Survival of Lactobacillus salivarius CECT 4063 and Stability of Antioxidant Compounds in Dried Apple Snacks as Affected by the Water Activity, the Addition of Trehalose and High Pressure Homogenization. Microorganisms 2020, 8, 1095. [Google Scholar] [CrossRef]
- Kopjar, M.; Ivić, I.; Buljeta, I.; Ćorković, I.; Vukoja, J.; Šimunović, J.; Pichler, A. Volatiles and Antioxidant Activity of Citrus Fiber/Blackberry Gels: Influence of Sucrose and Trehalose. Plants 2021, 10, 1640. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, Y.; Xiao, G.; Xu, Y.; Wua, J.; Tang, D.; Zhang, Y. Comparing product stability of probiotic beverages using litchi juice treated by high hydrostatic pressure and heat as substrates. Innov. Food Sci. Emerg. Technol. 2014, 23, 61–67. [Google Scholar] [CrossRef]
- Kuskoski, M.; Asuero, A.; Troncoso, A. Aplicación de diversos métodos químicos para determinar la capacidad antioxidante en pulpa de frutos. Ciência Tecnol. Aliment. 2005, 25, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Tao, Y.; Li, D.; Wen, G.; Zhou, J.; Manickam, S.; Han, Y.; Chai, W.S. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere 2021, 276, 130090. [Google Scholar] [CrossRef]
- Ferrari, G.; Maresca, P.; Ciccarone, R. The application of high hydrostatic pressure for the stabilization of functional foods: Pomegranate juice. J. Food Eng. 2010, 100, 245–253. [Google Scholar] [CrossRef]
- Kopjar, M.; Pichler, A.; Turi, J.; Piližota, V. Influence of trehalose addition on antioxidant activity, colour and texture of orange jelly during storage. Int. J. Food Sci. Technol. 2016, 51, 2640–2646. [Google Scholar] [CrossRef]
- Muñoz-Provencio, D.; Llopis, M.; Antolín, M.; de Torres, I.; Guarner, F.; Pérez-Martínez, G.; Monedero, V. Adhesion Properties of Lactobacillus casei Strains to Resected Intestinal Fragments and Components of the Extracellular Matrix. Arch. Microbiol. 2009, 191, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Rokana, N.; Singh, B.; Thakur, N.; Sharma, C.; Gulhane, R.; Panwar, H. Screening of cell surface properties of potential probiotic lactobacilli isolated from human milk. J. Dairy Res 2018, 85, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Perea Vélez, M.; De Keersmaecker, S.C.J.; Vanderleyden, J. Adherence Factors of Lactobacillus in the Human Gastrointestinal Tract. FEMS Microbiol. Lett. 2007, 276, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorca, G.; Torino, M.I.; Font de Valdez, G.; Ljungh, A. Lactobacilli express cell surface proteins which mediate binding of immobilized collagen and fibronectin. FEMS Microbiol. Lett. 2002, 206, 31–37. [Google Scholar] [CrossRef]
- Yadav, A.K.; Tyagi, A.; Kaushik, J.K.; Saklani, A.C.; Grover, S.; Batish, V.K. Role of Surface Layer Collagen Binding Protein from Indigenous Lactobacillus plantarum 91 in Adhesion and Its Anti-Adhesion Potential against Gut Pathogen. Microbiol. Res. 2013, 168, 639–645. [Google Scholar] [CrossRef]
- Celebioglu, H.U.; Olesen, S.V.; Prehn, K.; Lahtinen, S.J.; Brix, S.; Hachem, M.A.; Svensson, B. Mucin- and carbohydrate- stimulated adhesion and subproteome changes of the probiotic bacterium Lactobacillus acidophilus NCFM. J. Proteom. 2017, 163, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Patrignani, F.; Siroli, L.; Lanciotti, D.I.S. Potential of High Pressure Homogenization and Functional Strains for the Development of Novel Functional Dairy Foods. In Technological Approaches for Novel Applications in Dairy Processing; Koca, N., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, S.M.B.; Khaneghah, A.M.; Barba, F.J.; Nemati, Z.; Shokofti, S.S.; Alizadeh, F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. J. Funct. Foods 2017, 38, 409–414. [Google Scholar] [CrossRef]





| Treatment | Interval | ||||
|---|---|---|---|---|---|
| 0–24 h | 24–48 h | 24–72 h | 24–96 h | ||
| non-HPH | 0%T | 2.54 ± 0.06 dD | −1.13 ± 0.05 dC | −2.26 ± 0.05 aB | −2.400 ± 0.014 abA |
| 10%T | 2.33 ± 0.10 cD | −1.48 ± 0.10 cC | −1.81 ± 0.16 bB | −2.13 ± 0.06 cA | |
| I+HPH | 0%T | 1.70 ± 0.07 aD | −1.70 ± 0.14 bC | −2.11 ± 0.08 aB | −2.51 ± 0.14 aA |
| 10%T | 1.68 ± 0.15 aC | −2.08 ± 0.13 aB | −2.18 ± 0.07 aAB | −2.32 ± 0.03 bA | |
| HPH | 0%T | 2.68 ± 0.07 eC | −1.70 ± 0.04 bB | −1.80 ± 0.03 bB | −2.05 ± 0.08 cA |
| 10%T | 2.153 ± 0.010 bC | −1.16 ± 0.09 dB | −1.3 ± 0.2 cB | −1.52 ± 0.04 dA | |
| t (h) | Total Phenols (mg GAE/mL) | Total Flavonoids (mg QE/mL) | AO_DPPH (mg TE/mL) | AO_ABTS (mg TE/mL) | ||
|---|---|---|---|---|---|---|
| no HPH | 0%T | 0 | 0.84 ± 0.08 aAB | 0.747 ± 0.014 aA | 0.33 ± 0.02 aA | 1.35 ± 0.06 dD |
| 24 | 1.81 ± 0.13 dC | 2.09 ± 0.03 bcD | 0.51 ± 0.04 cB | 1.61 ± 0.08 cE | ||
| 48 | 0.99 ± 0.09 bB | 1.65 ± 0.07 cC | 0.31 ± 0.04 abA | 0.92 ± 0.05 bC | ||
| 72 | 0.86 ± 0.06 abAB | 1.64 ± 0.07 cC | 0.32 ± 0.03 cA | 0.85 ± 0.04 bB | ||
| 96 | 0.75 ± 0.09 aA | 1.130 ± 0.002 bB | 0.309 ± 0.012 cA | 0.69 ± 0.07 bA | ||
| 10%T | 0 | 0.90 ± 0.05 bB | 0.89 ± 0.04 bA | 0.31 ± 0.03 aAB | 1.34 ± 0.09 cdC | |
| 24 | 2.00 ± 0.10 eC | 2.19 ± 0.11 cD | 0.49 ± 0.02 bcC | 1.53 ± 0.02 cD | ||
| 48 | 0.79 ± 0.03 aAB | 1.4 ± 0.2 aC | 0.34 ± 0.05 bB | 0.78 ± 0.03 aB | ||
| 72 | 0.78 ± 0.03 aA | 1.15 ± 0.06 aB | 0.310 ± 0.011 cAB | 0.719 ± 0.014 aA | ||
| 96 | 0.7 ± 0.2 aA | 1.10 ± 0.03 aB | 0.28 ± 0.03 bA | 0.678 ± 0.005 abA | ||
| I + HPH | 0%T | 0 | 0.81 ± 0.04 aA | 0.747 ± 0.014 aA | 0.33 ± 0.02 aD | 1.26 ± 0.04 bcC |
| 24 | 1.5 ± 0.2 cD | 1.99 ± 0.09 bC | 0.37 ± 0.03 aE | 1.35 ± 0.06 bD | ||
| 48 | 1.37 ± 0.13 cCD | 2.00 ± 0.05 dC | 0.28 ± 0.03 aC | 1.23 ± 0.05 dC | ||
| 72 | 1.26 ± 0.04 cBC | 1.87 ± 0.14 dBC | 0.18 ± 0.02 aB | 1.11 ± 0.07 cB | ||
| 96 | 1.18 ± 0.05 cB | 1.8 ± 0.2 dB | 0.116 ± 0.006 aA | 0.76 ± 0.07 bA | ||
| 10%T | 0 | 0.90 ± 0.05 bA | 0.89 ± 0.04 bA | 0.31 ± 0.03 aC | 1.19 ± 0.08 bB | |
| 24 | 1.03 ± 0.14 bB | 1.65 ± 0.02 aD | 0.46 ± 0.03 bD | 1.38 ± 0.13 bC | ||
| 48 | 0.97 ± 0.07 bAB | 1.56 ± 0.06 bcC | 0.29 ± 0.02 aC | 1.14 ± 0.02 cB | ||
| 72 | 0.96 ± 0.09 bAB | 1.55 ± 0.03 bBC | 0.214 ± 0.008 bB | 1.1 ± 0.2 cB | ||
| 96 | 0.94 ± 0.05 bAB | 1.50 ± 0.05 cB | 0.119 ± 0.003 aA | 0.69 ± 0.03 bA | ||
HPH | 0%T | 0 | 0.84 ± 0.04 aA | 0.99 ± 0.02 dA | 0.30 ± 0.01 aA | 0.83 ± 0.04 aC |
| 24 | 0.83 ± 0.03 aA | 1.58 ± 0.10 aC | 0.51 ± 0.07 cD | 0.88 ± 0.03 aC | ||
| 48 | 0.80 ± 0.06 aA | 1.44 ± 0.12 abB | 0.47 ± 0.03 cCD | 0.82 ± 0.11 aC | ||
| 72 | 0.81 ± 0.06 aA | 1.44 ± 0.09 bB | 0.41 ± 0.03 dBC | 0.69 ± 0.08 aB | ||
| 96 | 0.75 ± 0.07 aA | 1.06 ± 0.06 aA | 0.39 ± 0.06 dB | 0.59 ± 0.10 aA | ||
| 10%T | 0 | 0.80 ± 0.04 aC | 0.94 ± 0.04 cA | 0.33 ± 0.03 aA | 0.80 ± 0.06 aB | |
| 24 | 0.82 ± 0.05 aC | 1.6 ± 0.2 aC | 0.50 ± 0.02 cD | 0.88 ± 0.05 aC | ||
| 48 | 0.79 ± 0.08 aBC | 1.53 ± 0.14 bcC | 0.47± 0.04 cC | 0.79 ± 0.03 aAB | ||
| 72 | 0.72 ± 0.05 aA | 1.45 ± 0.06 bC | 0.426 ± 0.014 dB | 0.73 ± 0.07 aAB | ||
| 96 | 0.72 ± 0.05 aAB | 1.20 ± 0.04 aB | 0.359 ± 0.013 dA | 0.72 ± 0.08 bA |
| Treatment | Total Phenols | Total Flavonoids | AO_DPPH | AO_ABTS | |
|---|---|---|---|---|---|
| non-HPH | 0%T | −23.1 ± 0.8 b | −7.1 ± 0.6 c | 55 ± 11 a | −36 ± 3 a |
| 10%T | −15 ± 8 c | −8 ± 2 c | 155 ± 19 c | −27 ± 4 b | |
| I+HPH | 0%T | −39 ± 2 a | −21.0 ± 1.2 a | 107 ± 15 b | −24 ± 3 b |
| 10%T | −14 ± 2 c | −14.3 ± 0.7 b | 117 ± 13 b | −27 ± 4 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burca-Busaga, C.G.; Betoret, N.; Seguí, L.; Barrera, C. Fermentation of Clementine Juice with Lactobacillus salivarius spp. salivarius CECT 4063: Effect of Trehalose Addition and High-Pressure Homogenization on Antioxidant Properties, Mucin Adhesion, and Shelf Life. Fermentation 2022, 8, 642. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8110642
Burca-Busaga CG, Betoret N, Seguí L, Barrera C. Fermentation of Clementine Juice with Lactobacillus salivarius spp. salivarius CECT 4063: Effect of Trehalose Addition and High-Pressure Homogenization on Antioxidant Properties, Mucin Adhesion, and Shelf Life. Fermentation. 2022; 8(11):642. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8110642
Chicago/Turabian StyleBurca-Busaga, Cristina Gabriela, Noelia Betoret, Lucía Seguí, and Cristina Barrera. 2022. "Fermentation of Clementine Juice with Lactobacillus salivarius spp. salivarius CECT 4063: Effect of Trehalose Addition and High-Pressure Homogenization on Antioxidant Properties, Mucin Adhesion, and Shelf Life" Fermentation 8, no. 11: 642. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8110642