Bioethanol Production as an Alternative End for Maple Syrups with Flavor Defects
Abstract
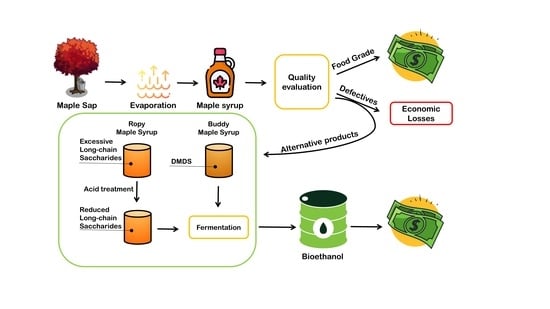
:1. Introduction
2. Materials and Method
2.1. Maple Syrups
2.2. Physical-Chemical Characterization of Maple Syrups
2.3. Acid Hydrolysis of Ropy Maple Syrup
2.4. Fermentation Conditions
2.5. Reducing Sugars Quantification
2.6. Sugar Profile
2.7. Organic Acids and Ethanol Quantification
2.8. Size-Exclusion Chromatography
2.9. Fermentation Parameters and Statistical Analysis
3. Results
3.1. Initial Characterization
3.2. Hydrolysis
3.3. Ethanol Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canada Statistical Overview of the Canadian Maple Industry. Available online: https://agriculture.canada.ca/en/canadas-agriculture-sectors/horticulture/horticulture-sector-reports/statistical-overview-canadian-maple-industry-2020 (accessed on 10 December 2021).
- Producteurs et Productrices Acéricoles du Québec 2020 Activity Report. Available online: https://ppaq.ca/en/our-publications/annual-reports/ (accessed on 10 December 2021).
- Acer Division Inspection Inc. Vérification de la qualité du sirop d’érable en vrac. Available online: https://gestion.centreacer.qc.ca/fr/UserFiles/Documents/ppaq_depliantacer_infographics_2021-final.pdf (accessed on 10 December 2021).
- Caughron, A.; Legault, S.; Haut, C.; Houle, D.; Reynolds, T.W. A Changing Climate in the Maple Syrup Industry: Variation in Canadian and U.S.A. Producers’ Climate Risk Perceptions and Willingness to Adapt Across Scales of Production. Small-Scale For. 2020, 20, 73–95. [Google Scholar] [CrossRef]
- Jennifer, J.P.; Maria, C.F. A Comprehensive Review of Maple Sap Microbiota and Its Effect on Maple Syrup Quality. Food Rev. Int. 2020, 1–20. [Google Scholar] [CrossRef]
- Camara, M.; Cournoyer, M.; Sadiki, M.; Martin, N. Characterization and Removal of Buddy Off-Flavor in Maple Syrup. J. Food Sci. 2019, 84, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- N’guyen, G.Q.; Martin, N.; Jain, M.; Lagacé, L.; Landry, C.R.; Filteau, M. A systems biology approach to explore the impact of maple tree dormancy release on sap variation and maple syrup quality. Sci. Rep. 2018, 8, 14658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose Garcia, E.; McDowell, T.; Ketola, C.; Jennings, M.; David Miller, J.; Renaud, J.B. Metabolomics reveals chemical changes in Acer saccharum sap over a maple syrup production season. PLoS ONE 2020, 15, e0235787. [Google Scholar] [CrossRef]
- Pelletier, M.; Lagacé, L. Forêts de Chez Nous. 2018, pp. 1–5. Available online: https://ppaq.ca/en/our-publications/forets-de-chez-nous-magazine/ (accessed on 25 January 2022).
- Britten, M.; Morin, A. Functional characterization of the exopolysaccharide from enterobacter agglomerans grown on low-grade maple sap. LWT Food Sci. Technol. 1995, 28, 264–271. [Google Scholar] [CrossRef]
- Fabian, F.W.; Buskirk, H.H. Aërobacter aërogenes as a Cause of Ropiness in Maple Sirup. Ind. Eng. Chem. 1935, 27, 349–350. [Google Scholar] [CrossRef]
- Lagacé, L.; Camara, M.; Leclerc, S.; Charron, C.; Sadiki, M. Chemical and microbial characterization of ropy maple sap and syrup. Maple Syrup Dig. 2018, 9–19. [Google Scholar]
- Règlement des Producteurs Acéricoles sur les Normes de Qualité et le Classement; Government of Quebec (CA): Québec, QC, Canada, 2021; pp. 1–10.
- Ball, D.W. The Chemical Composition of Maple Syrup. J. Chem. Educ. 2007, 84, 1647–1650. [Google Scholar] [CrossRef]
- Stuckel, J.G.; Low, N.H. The chemical composition of 80 pure maple syrup samples produced in North America. Food Res. Int. 1996, 29, 373–379. [Google Scholar] [CrossRef]
- Heiligmann, R.B.; Koelling, M.R.; Perkins, T.D. (Eds.) North American Maple Syrup Producers Manual, 2nd ed.; The Ohio State University: Columbus, OH, USA, 2006. [Google Scholar]
- He, M.; Qin, H.; Yin, X.; Ruan, Z.; Tan, F.; Wu, B.; Shui, Z.; Dai, L.; Hu, Q. Direct ethanol production from dextran industrial waste water by Zymomonas mobilis. Korean J. Chem. Eng. 2014, 31, 2003–2007. [Google Scholar] [CrossRef]
- Kim, J.H.; Ryu, J.; Huh, I.Y.; Hong, S.-K.; Kang, H.A.; Chang, Y.K. Ethanol production from galactose by a newly isolated Saccharomyces cerevisiae KL17. Bioprocess Biosyst. Eng. 2014, 37, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, S.; Ando, H.; Ariga, O.; Sakuma, Y. Direct ethanol production from cellulosic materials by consolidated biological processing using the wood rot fungus Schizophyllum commune. Bioresour. Technol. 2015, 197, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Gouvernement du Québec 2030 Plan for a Green Economy. Available online: https://www.quebec.ca/en/government/policies-orientations/plan-green-economy (accessed on 10 December 2021).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Demeke, M.M.; Dietz, H.; Li, Y.; Foulquié-Moreno, M.R.; Mutturi, S.; Deprez, S.; Den Abt, T.; Bonini, B.M.; Liden, G.; Dumortier, F.; et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol. Biofuels 2013, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Beigbeder, J.-B.; de Medeiros Dantas, J.M.; Lavoie, J.-M. Optimization of Yeast, Sugar and Nutrient Concentrations for High Ethanol Production Rate Using Industrial Sugar Beet Molasses and Response Surface Methodology. Fermentation 2021, 7, 86. [Google Scholar] [CrossRef]
- Gad, H.A.; Ramadan, M.F.; Farag, M.A. Authentication and quality control determination of maple syrup: A comprehensive review. J. Food Compos. Anal. 2021, 100, 103901. [Google Scholar] [CrossRef]
- Mohammed, F.; Sibley, P.; Guillaume, D.; Abdulwali, N. Chemical composition and mineralogical residence of maple syrup: A comprehensive review. Food Chem. 2022, 374, 131817. [Google Scholar] [CrossRef]
- Sun, J.; Ma, H.; Seeram, N.P.; Rowley, D.C. Detection of Inulin, a Prebiotic Polysaccharide, in Maple Syrup. J. Agric. Food Chem. 2016, 64, 7142–7147. [Google Scholar] [CrossRef] [Green Version]
- Brochu, M.; Lafrance, C.P.; Landry, E.; Maheux, M. Isolation and characterization of major polysaccharides from maple sugar. Bioact. Carbohydrates Diet. Fibre 2019, 17, 100174. [Google Scholar] [CrossRef]
- Yan, X.; Evenocheck, H.M. Chitosan analysis using acid hydrolysis and HPLC/UV. Carbohydr. Polym. 2012, 87, 1774–1778. [Google Scholar] [CrossRef]
- Smits, J.; van Haastert, M.; Janse, A.M.C.; Maas, J.; de Graaf, K.; Kroon, H.; Verlinden, R.A.J.; Happel, A. Scale-up of optimal mild-acid pretreatment conditions in the production and application of lignocellulosic sugars from wood. Bioresour. Technol. Rep. 2020, 9, 100361. [Google Scholar] [CrossRef]
- Taga, A.; Kodama, S. Analysis of reducing carbohydrates and fructosyl saccharides in maple syrup and maple sugar by CE. Chromatographia 2012, 75, 1009–1016. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Madl, R.L.; Saida, L.; Abeykoon, J.P. Ethanol production from orange peels: Twostage hydrolysis and fermentation studies using optimized parameters through experimental design. J. Agric. Food Chem. 2010, 58, 3422–3429. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Hu, R.; Shupe, A.; Lin, L.; Liang, B. A sustainable woody biomass biorefinery. Biotechnol. Adv. 2012, 30, 785–810. [Google Scholar] [CrossRef]
- Jeong, H.S.; Jang, S.K.; Kim, H.Y.; Yeo, H.; Choi, J.W.; Choi, I.G. Effect of freeze storage on hemicellulose degradation and enzymatic hydrolysis by dilute-acid pretreatment of Mongolian oak. Fuel 2016, 165, 145–151. [Google Scholar] [CrossRef]
- Huang, H.J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B.V. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Kupiainen, L.; Ahola, J.; Tanskanen, J. Kinetics of glucose decomposition in formic acid. Chem. Eng. Res. Des. 2011, 89, 2706–2713. [Google Scholar] [CrossRef]
- Barbosa, H.S.; de Abreu Silveira, E.; Miranda, M.; Ernandes, J.R. Efficient very-high-gravity fermentation of sugarcane molasses by industrial yeast strains. J. Inst. Brew. 2016, 122, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Vučurović, V.M.; Puškaš, V.S.; Miljić, U.D. Bioethanol production from sugar beet molasses and thick juice by free and immobilised Saccharomyces cerevisiae. J. Inst. Brew. 2018, 125, 134–142. [Google Scholar] [CrossRef]
- Darvishi, F.; Moghaddami, N.A. Optimization of an industrial medium from molasses for bioethanol production using the Taguchi statistical experimental-design method. Fermentation 2019, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Klasson, K.T.; Boone, S.A. Bioethanol fermentation of clarified sweet sorghum (Sorghum bicolor (L.) Moench) syrups sealed and stored under vegetable oil. Ind. Crop. Prod. 2021, 163, 113330. [Google Scholar] [CrossRef]
- Lamas, A.; Miranda, J.; Vázquez, B.; Cepeda, A.; Franco, C. An Evaluation of Alternatives to Nitrites and Sulfites to Inhibit the Growth of Salmonella enterica and Listeria monocytogenes in Meat Products. Foods 2016, 5, 74. [Google Scholar] [CrossRef] [PubMed]




| Parameter | MS | RMS | BMS |
|---|---|---|---|
| Soluble solids (°Bx) | 67.80 ± 0.50 | 66.23 ± 0.41 | 66.70 ± 0.32 |
| Density (g/mL) | 1.31 ± 0.01 | 1.32 ± 0.01 | 1.32 ± 0.01 |
| Viscosity (cP) | 130.55 ± 3.96 | 427.79 ± 7.57 | 172.49 ± 7.91 |
| Lactic Acid (g/L) | 1.20 ± 0.02 | 3.75 ± 0.05 | 2.77 ± 0.04 |
| Acetic Acid (g/L) | 2.94 ± 0.01 | 1.53 ± 0.01 | 3.70 ± 0.02 |
| Sucrose (g/L) | 577.49 ± 0.15 | 538.99 ± 1.84 | 542.75 ± 5.39 |
| Glucose (g/L) | 2.60 ± 0.54 | 8.43 ± 0.85 | 11.12 ± 0.95 |
| Fructose (g/L) | 1.92 ± 0.01 | 6.99 ± 0.05 | 5.12 ± 0.10 |
| pH | 7.11 ± 0.01 | 7.72 ± 0.02 | 7.10 ± 0.01 |
| Protein (g/L) | 0.35 ± 0.07 | 0.29 ± 0.02 | 0.35 ± 0.03 |
| Media | Ethanol Conc. (g/L) | Ethanol % (v/v) | YP/S, Yield Coeff. (g/g) | Productivity (g/L/h) | Substrate Utilization Rate (g/L/h) | Efficiency (%) |
|---|---|---|---|---|---|---|
| MS | 88.69 ± 12.73 | 11.24 ± 1.61 | 0.52 ± 0.01 | 0.92 ± 0.13 | 1.76 ± 0.22 | 95.34 ± 6.46 |
| RMS | 74.92 ± 5.78 | 9.49 ± 0.73 | 0.51 ± 0.00 | 0.78 ± 0.06 | 1.53 ± 0.12 | 90.08 ± 9.41 |
| RMS10 | 72.53 ± 12.29 | 9.19 ± 1.56 | 0.51 ± 0.00 | 0.76 ± 0.13 | 1.47 ± 0.24 | 87.86 ± 14.69 |
| RMS5 | 89.16 ± 1.07 | 11.30 ± 0.14 | 0.53 ± 0.04 | 0.93 ± 0.01 | 1.74 ± 0.13 | 95.66 ± 4.88 |
| BMS | 86.44 ± 9.24 | 10.96 ± 1.17 | 0.50 ± 0.07 | 0.90 ± 0.10 | 1.80 ± 0.06 | 93.34 ± 9.41 |
| YPD | 60.56 ± 9.62 | 7.67 ± 1.22 | 0.41 ± 0.05 | 0.63 ± 0.10 | 1.52 ± 0.09 | 78.38 ± 10.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Medeiros Dantas, J.M.; Álvares Monge Neto, A.; Ghislain, T.; Lavoie, J.-M. Bioethanol Production as an Alternative End for Maple Syrups with Flavor Defects. Fermentation 2022, 8, 58. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8020058
de Medeiros Dantas JM, Álvares Monge Neto A, Ghislain T, Lavoie J-M. Bioethanol Production as an Alternative End for Maple Syrups with Flavor Defects. Fermentation. 2022; 8(2):58. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8020058
Chicago/Turabian Stylede Medeiros Dantas, Julia Maria, André Álvares Monge Neto, Thierry Ghislain, and Jean-Michel Lavoie. 2022. "Bioethanol Production as an Alternative End for Maple Syrups with Flavor Defects" Fermentation 8, no. 2: 58. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8020058