Acquisition, Characterization, and Optimization of Distilled Bioethanol Generated from Fermented Carrot (Daucus carota) Residues
Abstract
:1. Introduction
2. Materials and Methods
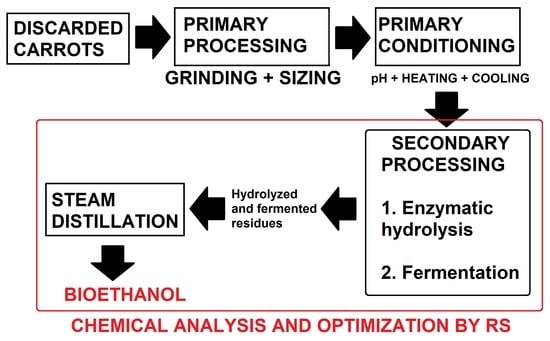
2.1. Biomass Collection and Analysis of Raw Material
2.2. Bioethanol Acquisition I: Enzymatic Hydrolysis
2.3. Bioethanol Acquisition II: Alcoholic Fermentation
2.4. Determination of TSS and Alcohol Content in Distilled Product
2.5. Statistical Analysis and Response Surface
2.6. Chemical Analysis
- Relative density and viscosity were performed according to ASTM D4052-04 [31], which covers the determination of density, relative density, and API gravity of petroleum distillates and viscous oils, using a bulb density meter. Relative viscosity was measured in a U-type viscometer as the difference in flow time for water flow.
- Aldehyde, carboxylic acid, and ketone analyses were performed using an 8860 GC gas chromatograph (Agilent, Wilmington, DE, USA), equipped with an FID Headspace detector and a 30 m × 0.25 mm packed column. The procedure was performed based on Medina et al. [32] for the determination of volatiles in alcoholic beverages. The conductive gas was hydrogen (flow rate of 1.5 mL/min). The temperature was programmed gradually from 50 °C (1 min), with a gradual increase of 4 °C/min up to 180 °C, and 8 °C/min up to 250 °C, with final maintenance for 5 min. Acetaldehyde, acetic acid, and acetone were used as standard.Aldehyde concentration was expressed as mg of acetaldehyde per 100 mL of anhydrous alcohol (AA). Carboxylic acid concentration was expressed as mg of acetic acid per 100 mL AA and ketones as parts per million (ppm).
- 3.
- Superior alcohol and methanol analyses were performed using the same chromatograph and column as in the previous section. The analysis was guided according to the European standard BS EN 1572:2013 [33] and Zhou et al. [34]. The conductive gas was helium of high purity (flow rate of 1.0 mL/min). The temperature was programmed gradually from 40 °C (2 min), with a gradual increase of 5 °C/min, up to 230 °C, with final holding for 3 min. The intensity of the individual peaks detected was related to the GC reference library to methanol, 1-propanol, 1-butanol, 2-butanol, isobutanol, 2-methylbutanol, and 3-methylbutanol.
- 4.
- Total phenol content was determined using the Folin−Ciocalteu spectrophotometric method compared with a standard gallic acid (GAE) curve as a reference. A quantity of 2 mL of the bioethanol was transferred to a flask and made up to 10 mL with a 10.75% (m/v) ethanolic solution of Na2CO3 and 1 mL of Folin−Ciocalteu reagent [35]. The sample was scanned in a UV/vis spectrophotometer at 760 nm; its absorbance was determined through the reference curve and reported as g/mL of solution.
2.7. Lower Heating Value (LHV)
3. Results and Discussion
3.1. Raw Material Characterization
3.2. Bioethanol Acquistion
3.3. Statistical Analysis and Response Surface
3.4. Bioethanol Chemical Analysis
3.5. Energy Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, R.; Sorrell, S. The future of oil supply. Phil. Trans. R. Soc. 2014, 372, 20130179. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Klingmüller, K.; Pozzer, A.; Burnett, R.; Haines, A.; Ramanathan, V. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl. Acad. Sci. USA 2019, 116, 7192–7197. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Dhungana, P.; Prajapati, B.; Maharjan, S.; Joshi, J. Current Trends in Lignocellulosic Bioethanol Production. Int. J. Appl. Sci. Biotechnol. 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Jarboe, L.; Wen, Z.; Choi, D.; Brown, R. Hybrid thermochemical processing: Fermentation of pyrolysis-derived bio-oil. Appl. Microbiol. Biotechnol. 2011, 91, 1519–1523. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Mohammadi, A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Khandaker, M.; Qiamuddin, K.; Majrashi, A.; Dalorima, T. Bio-Ethanol Production from Fruit and Vegetable Waste by Using Saccharomyces cerevisiae. In Bioethanol Technologies; Intech-Open: KwaZulu-Natal, Sudáfrica, 2018. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, J.; Baskar, C. Lignocellulosic biomass for bioethanol production through microbes: Strategies to improve process efficiency. Prospect. Renew. Bioprocess. Future Energy Syst. 2019, 10, 357–386. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Luo, L.; Zhang, F.; Yi, Y.; Shan, Y.; Lü, X. A review on recycling techniques for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2021, 149, 111370. [Google Scholar] [CrossRef]
- Bender, L.; Lopes, S.; Gomes, K.; Devos, R.; Colla, L. Challenges in bioethanol production from food residues. Bioresour. Technol. Rep. 2022, 19, 101171. [Google Scholar] [CrossRef]
- Khan, O.; Yadav, A.; Khan, M.; Parvez, M. Characterization of bioethanol obtained from Eichhornia Crassipes plant; its emission and performance analysis on CI engine. Energy Sources Part A 2021, 43, 1793–1803. [Google Scholar] [CrossRef]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from lignocellulosic biomass: Current findings determine research priorities. Sci. World J. 2014, 1, 298153. [Google Scholar] [CrossRef] [PubMed]
- Milano, J.; Ong, H.; Masjuki, H.; Silitonga, A.; Kusumo, F.; Dharma, S.; Sebayang, A.; Cheah, M.; Wang, C. Physicochemical property enhancement of biodiesel synthesis from hybrid feedstocks of waste cooking vegetable oil and beauty leaf oil through optimized alkaline-catalysed transesterification. Waste Manag. 2018, 80, 435–449. [Google Scholar] [CrossRef]
- Candama, M.; Duque, S.; Cadena, E. Optimization of enzymatic pretreatments to obtain fermentable sugars from fruit and vegetable waste. Waste Biomass-Valorization 2020, 11, 5991–6002. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.; Boyce, A.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sust. En. Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Anwar, M.; Ma, H.; Yue, S.; Wang, Q.; Tu, M. Concise review on ethanol production from food waste: Development and sustainability. Environ. Sci. Pollut. Res. 2018, 25, 28851–28863. [Google Scholar] [CrossRef]
- Panahi, H.; Dehhaghi, M.; Guillemin, G.; Gupta, V.; Lam, S.; Aghbashlo, M.; Tabatabaei, M. Bioethanol production from food wastes rich in carbohydrates. Curr. Opin. Food Sci. 2022, 43, 71–81. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.; Serwanska, K. Bioethanol Production from Lignocellulosic Biomass— Challenges and Solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Vicente, G.; Coteron, A.; Martinez, M.; Aracil, J. Application of the factorial design of experiments and response surface methodology to optimize biodiesel production. Ind. Crops Prod. 1998, 8, 29–35. [Google Scholar] [CrossRef]
- Manojkumar, N.; Muthukumaran, C.; Sharmila, G. A comprehensive review on the application of response surface methodology for optimization of biodiesel production using different oil sources. J. King Saud Univ. Eng. Sci. 2020, 34, 198–208. [Google Scholar] [CrossRef]
- De Vrije, T.; Budde, M.; Lips, S.; Bakker, R.; Mars, A.; Claassen, P. Hydrogen production from carrot pulp by the extreme thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int. J. Hydrog. Energy 2010, 35, 13206–13213. [Google Scholar] [CrossRef]
- Aimaretti, N.; Clementz, A.; Codevilla, A.; Rojas, M.; Yori, J. Sustainable fermentation processing of two revalorized agro-industrial discards: Carrot and brewer’s yeast. Int. J. Energy Environ. Eng. 2013, 4, 24. [Google Scholar] [CrossRef]
- Aimaretti, N.; Ybalo, C. Valorization of carrot and yeast discards for the obtention of ethanol. Biomass-Bioenergy 2012, 42, 18–23. [Google Scholar] [CrossRef]
- Demiray, E.; Karatay, S.; Dönmez, S.; Dönmez, G. The usage of carrot pomace for bioethanol production. J. Chil. Chem. Soc. 2016, 61, 2996–2998. [Google Scholar] [CrossRef]
- Khoshkho, S.; Mahdavian, M.; Karimi, F.; Karimi-Maleh, H.; Razaghi, P. Production of bioethanol from carrot pulp in the presence of Saccharomyces cerevisiae and beet molasses inoculum; a biomass based investigation. Chemosphere 2022, 286, 131688. [Google Scholar] [CrossRef]
- Yu, C.; Jiang, B.; Duan, K. Production of bioethanol from carrot pomace using the thermotolerant yeast Kluyveromyces marxianus. Energies 2013, 6, 1794–1801. [Google Scholar] [CrossRef]
- Ramos-Andrés, M.; Aguilera-Torre, B.; García-Serna, J. Hydrothermal production of high-molecular weight hemicellulose-pectin, free sugars and residual cellulose pulp from discarded carrots. J. Clean. Prod. 2021, 290, 125179. [Google Scholar] [CrossRef]
- Del Águila, N.; Mendocilla, A.; Villalobos, V. Evaluación por el método de superficie de respuesta del efecto de la temperatura y tiempo de transesterificación en el rendimiento y poder calórico del biodiesel obtenido a partir del aceite de piñón (Jatropha curcas). Agroindust. Sci. 2011, 1, 63–72. [Google Scholar] [CrossRef]
- Ngomade, S.; Tchuifon, R.; Tagne, R.; Ngueteu, M.; Patai, H.; Nche, G.; Anagho, S. Optimization by Response Surface Methodology of Biodiesel Production from Podocarpus falcatus Oil as a Cameroonian Novel Nonedible Feedstock. J. Chem. 2022, 1, 3786602. [Google Scholar] [CrossRef]
- Aimaretti, N.; Ybalo, C.; Rojas, M.; Plou, F.; Yori, J. Production of bioethanol from carrot discards. Bioresour. Technol. 2012, 123, 727–732. [Google Scholar] [CrossRef]
- D4052-04; Standard Test Method for Density, Relative Density, and API Gravity of Liquids. ASTM International: West Conshohocken, PA, USA, 2004.
- Medina, G.; Juárez, R.; Peña, A. Identification and Quantification of Aldehydes in Mezcal by Solid Phase Microextraction with On-fiber Derivatization-Gas Cromatography. J. Mex. Chem. Soc. 2011, 55, 84–88. [Google Scholar]
- European Standard [ES] IP 571; Ethanol as a Blending Component for Petrol—Determination of Higher Alcohols, Methanol and Other Impurities—Gas Chromatographic Method. European Committee for Standardization: Brussels, Belgium, 2013.
- Zhou, Z.; Ni, W.; Ji, Z.; Liu, S.; Han, X.; Li, X.; Mao, J. Development of a rapid method for determination of main higher alcohols in fermented alcoholic beverages based on dispersive liquid-liquid microextraction and gas chromatography-mass spectrometry. Food Anal. Meth. 2020, 13, 591–600. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.; De Mello, J. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- E711; Standard Test Method for Gross Calorific Value of Refuse-Derived Fuel by the Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2004.
- Tse, T.; Wiens, D.; Reaney, M. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Clementz, A.; Aimaretti, N.; Manuale, D.; Codevilla, A.; Yori, J. Optimization of ethanol fermentation from discarded carrots using immobilized Saccharomyces cerevisiae. Int. J. Energy Environ. Eng. 2015, 6, 129–135. [Google Scholar] [CrossRef]
- Das, N.; Jena, P.; Padhi, D.; Kumar, M.; Sahoo, G. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Convers. Biorefin. 2021, 13, 1503–1527. [Google Scholar] [CrossRef]
- Li, A.; Antizar, B.; Khraisheh, M. Bioconversion of municipal solid waste to glucose for bio-ethanol production. Bioproc. Biosyst. Eng. 2007, 30, 189–196. [Google Scholar] [CrossRef]
- Obeta, J.; Ossai, E.; Njoku, O. Optimization and characterization of bioethanol production from Abrus seed flour. Int. J. Energy Res. 2021, 45, 3883–3898. [Google Scholar] [CrossRef]
- Sánchez, C.; Santos, S.; Sánchez, R.; Lienemann, C.; Todolí, J. Profiling of Organic Compounds in Bioethanol Samples of Different Nature and the Related Fractions. ACS Omega 2020, 5, 20912–20921. [Google Scholar] [CrossRef]
- Tüccar, G.; Tosun, E.; Uludamar, E. Investigations of effects of density and viscosity of diesel and biodiesel fuels on NOx and other emission formations. J. Eng. Sci. 2018, 6, 81–85. [Google Scholar] [CrossRef]
- Todoruț, A.; Molea, A.; Barabás, I. Predicting the Temperature and Composition–Dependent Density and Viscosity of Diesel Fuel–Ethanol Blends. Period. Polytech. Chem. Eng. 2020, 64, 213–220. [Google Scholar] [CrossRef]
- Saka, A.; Afolabi, A.; Ogochukwu, M. Production and Characterization of Bioethanol from Sugarcane Bagasse as Alternative Energy Sources. World Congress on Engineering WCE 2015, pp. 876–880. Available online: http://www.iaeng.org/publication/WCE2015/WCE2015_pp876-880.pdf (accessed on 24 August 2023).
- Harrison, O.; Ekene, C.; Nathaniel, I. Production and Characterization of Bioethanol from Acid Catalysed Hydrolysis of Cellulosic Biomass (Maize Cob). J. Energy Environ. Chem. Eng. 2022, 7, 66–70. [Google Scholar]
- Rabiu, A.; Elinge, C.; Ambursa, M.; Rabiu, A.; Samaila, D. Characterization of Bioethanol Fuel from Rice and Corn Straws: A Comparative Study. Equity J. Sci. Technol. 2021, 8, 74–78. [Google Scholar] [CrossRef]
- Bashir, A.; Baba, N.; Akpomie, T. Process Optimization and Characterization of Bioethanol from Yam (Dioscorea rotundata) Peels. Int. J. Sci. Res. Pub. 2022, 12, 223–229. [Google Scholar] [CrossRef]
- Raj, T.; Kapoor, M.; Gaur, R.; Christopher, J.; Lamba, B.; Tuli, D.; Kumar, R. Physical and chemical characterization of various Indian agriculture residues for biofuels production. Energy Fuels 2015, 29, 3111–3118. [Google Scholar] [CrossRef]
- Bridgeman, T.; Jones, J.; Shield, I.; Williams, P. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Kumar, S.; Paritosh, K.; Pareek, N.; Chawade, A.; Vivekanand, V. De-construction of major Indian cereal crop residues through chemical pretreatment for improved biogas production: An overview. Renew. Sustain. Energy Rev. 2018, 90, 160–170. [Google Scholar] [CrossRef]
- Worasuwannarak, N.; Sonobe, T.; Tanthapanichakoon, W. Pyrolysis behaviors of rice straw, rice husk, and corncob by TG MS technique. J. Anal. Appl. Pyrolysis 2007, 78, 265–271. [Google Scholar] [CrossRef]
- Vassilev, S.; Baxter, D.; Andersen, L.; Vassileva, C. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Tsai, W.; Lee, M.; Chang, Y. Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J. Anal. Appl. Pyrolysis 2006, 76, 230–237. [Google Scholar] [CrossRef]
| Exp. | Time (min), X1 | WY/WE (gyeast/genzime), X2 | pH, X3 | TSS (°Brix), Y1 | Ethanol Content (%), Y2 |
|---|---|---|---|---|---|
| 1 | 180 | 6 | 5.0 | 6.73 | 82.26 |
| 2 | 120 | 12 | 5.5 | 6.87 | 82.89 |
| 3 | 120 | 12 | 4.5 | 6.86 | 82.92 |
| 4 | 180 | 15 | 6.0 | 6.85 | 83.37 |
| 5 | 180 | 15 | 4.0 | 6.83 | 83.44 |
| 6 | 240 | 12 | 5.5 | 6.67 | 83.48 |
| 7 | 240 | 12 | 4.5 | 6.66 | 83.51 |
| 8 | 60 | 15 | 5.0 | 6.92 | 83.82 |
| 9 | 180 | 15 | 5.0 | 6.72 | 84.41 |
| 10 | 180 | 15 | 5.0 | 6.71 | 84.42 |
| 11 | 180 | 15 | 5.0 | 6.72 | 84.43 |
| 12 | 300 | 15 | 5.0 | 6.52 | 84.99 |
| 13 | 120 | 18 | 5.5 | 6.83 | 85.05 |
| 14 | 120 | 18 | 4.5 | 6.82 | 85.08 |
| 15 | 240 | 18 | 5.5 | 6.63 | 85.63 |
| 16 | 240 | 18 | 4.5 | 6.62 | 85.67 |
| 17 | 180 | 24 | 5.0 | 6.60 | 88.73 |
| Variable | Sum of Squares | Df | Mean Squares | F-Relation | p-Value |
|---|---|---|---|---|---|
| Total Soluble Solids Content TSS (°Brix) | |||||
| Time (min)-X1 | 0.16 | 1 | 0.16 | 86092.60 | 0.0000 |
| WY/WE (g/g)-X2 | 0.00185548 | 1 | 0.00185548 | 998.40 | 0.0000 |
| pH-X3 | 0.02015460 | 1 | 0.02015460 | 10844.78 | 0.0000 |
| 0.00451098 | 1 | 0.00451098 | 2427.26 | 0.0000 | |
| 0.02056800 | 1 | 0.02056800 | 11067.22 | 0.0000 | |
| Total error | 0.00002044 | 11 | 0.00000186 | ||
| Total correlation | 0.205494 | 16 | |||
| R2 = 0.90 | R2 adjusted = 0.89 | ||||
| Ethanol content (%) | |||||
| Time-X1 | 0.05139200 | 1 | 0.0513920 | 1683.50 | 0.0000 |
| WY/WE (g/g)-X2 | 0.01318220 | 1 | 0.0131822 | 431.82 | 0.0000 |
| pH-X3 | 1.22437000 | 1 | 1.2243700 | 40107.98 | 0.0000 |
| 0.00026785 | 1 | 0.0002679 | 8.77 | 0.0142 | |
| 1.42777000 | 1 | 1.4277700 | 46770.84 | 0.0000 | |
| 1.23625000 | 1 | 1.2362500 | 40497.20 | 0.0000 | |
| Total error | 0.00030527 | 10 | 0.00003053 | ||
| Total correlation | 36.0878 | 16 | |||
| R2 = 0.95 | R2 adjusted = 0.90 | ||||
| Exp. | SST Theo. (°Brix) | SST Exp. (°Brix) | Relat. Deviation SST (%) | Alcohol Content Theo. (%) | Alcohol Content Exp. (%) | Relat. Deviation Alcohol Content (%) |
|---|---|---|---|---|---|---|
| 1 | 6.93 | 6.73 | 2.89 | 81.96 | 82.26 | 0.37 |
| 2 | 7.12 | 6.87 | 3.51 | 82.76 | 82.89 | 0.16 |
| 3 | 7.02 | 6.86 | 2.28 | 82.79 | 82.92 | 0.16 |
| 4 | 7.15 | 6.85 | 4.20 | 83.06 | 83.37 | 0.37 |
| 5 | 6.95 | 6.83 | 1.73 | 83.13 | 83.44 | 0.37 |
| 6 | 6.92 | 6.67 | 3.61 | 82.94 | 83.48 | 0.65 |
| 7 | 6.82 | 6.66 | 2.35 | 82.97 | 83.51 | 0.65 |
| 8 | 7.12 | 6.92 | 2.81 | 83.78 | 83.82 | 0.05 |
| 9 | 6.92 | 6.72 | 2.89 | 84.11 | 84.41 | 0.36 |
| 10 | 6.92 | 6.71 | 3.03 | 84.11 | 84.42 | 0.37 |
| 11 | 6.92 | 6.72 | 2.89 | 84.11 | 84.43 | 0.38 |
| 12 | 6.72 | 6.52 | 2.98 | 84.15 | 84.99 | 1.00 |
| 13 | 7.07 | 6.83 | 3.39 | 84.91 | 85.05 | 0.16 |
| 14 | 6.97 | 6.82 | 2.15 | 84.94 | 85.08 | 0.16 |
| 15 | 6.87 | 6.63 | 3.49 | 85.09 | 85.63 | 0.63 |
| 16 | 6.77 | 6.62 | 2.22 | 85.13 | 85.67 | 0.63 |
| 17 | 6.80 | 6.60 | 2.94 | 88.42 | 88.73 | 0.35 |
| Optimal | 6.40 | 6.20 | 3.13 | 89.30 | 92.48 | 3.56 |
| Parameter | Bioethanol (This Study) | Standard Ethanol | ASTM |
|---|---|---|---|
| Ethanol content (% v/v) on distilled product | 92.48 | - | >92.00 |
| Density at 15 °C (g/cm3) | 0.89 | 0.794 | 0.80 |
| Specific gravity | 0.90 | -- | -- |
| Viscosity at 40 °C (mm2/s) | 1.65 | 1.30 | 1.34 |
| Flashpoint (°C) of distilled product | 14.5 | 12.5 | 18.60 |
| Aldehydes (mg/100 mL) | 1.30 | -- | -- |
| Ketones (ppm) | 0.10 | -- | -- |
| Acidity (mg/100 mL acetic acid) | 0.90 | -- | >0.70 |
| Superior alcohols (mg/100 mL) | 2.30 | -- | -- |
| Methanol content (% m/m) | 0.01 | -- | <0.50 |
| Phenol−water ratio (g/mL) | 0.20 | -- | -- |
| Moisture content (%) | 2.50 | -- | 20.00 |
| Sugar content (% m/v) | 0.40 | -- | -- |
| Source | LHV (MJ/kg) | Reference |
|---|---|---|
| Rice husk | 14.9 | [49] |
| Wheat straw | 17.0–18.9 | [50,51] |
| Rice straw | 14.5–15.5 | [51,52] |
| Cotton stalk | 17.3 | [49] |
| Sorghum stalk | 16.9 | [49] |
| Corn stover | 16.2–16.5 | [51,53] |
| Mustard stalk | 15.9 | [49] |
| Sugarcane bagasse | 18.61–18.73 | [45,54] |
| Corn cob | 15.5 | [49] |
| Carrot residues | 23.82 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-Velásquez, A.; Quispe-Coquil, V.; Casimiro-Soriano, E.M.; Tapia-Zarate, K.M.; Huamán-De la Cruz, A.R. Acquisition, Characterization, and Optimization of Distilled Bioethanol Generated from Fermented Carrot (Daucus carota) Residues. Fermentation 2023, 9, 867. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation9100867
Palacios-Velásquez A, Quispe-Coquil V, Casimiro-Soriano EM, Tapia-Zarate KM, Huamán-De la Cruz AR. Acquisition, Characterization, and Optimization of Distilled Bioethanol Generated from Fermented Carrot (Daucus carota) Residues. Fermentation. 2023; 9(10):867. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation9100867
Chicago/Turabian StylePalacios-Velásquez, Abraham, Violeta Quispe-Coquil, Enzo Martín Casimiro-Soriano, Karla Milagros Tapia-Zarate, and Alex Rubén Huamán-De la Cruz. 2023. "Acquisition, Characterization, and Optimization of Distilled Bioethanol Generated from Fermented Carrot (Daucus carota) Residues" Fermentation 9, no. 10: 867. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation9100867