Genotype and Maturity Stage Affect the Content and Composition of Polyamines in Tomato—Possible Relations to Plant and Human Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growing Conditions and Sampling
2.2. Reagents
2.3. Fruit Weight and Firmness
2.4. Ascorbic Acid (Aa) Analysis
2.5. Color Measurement, Total Soluble Solids (Tss), pH, and Titratable Acidity (TA)
2.6. Polyamines Analysis
2.7. Statistical Analysis
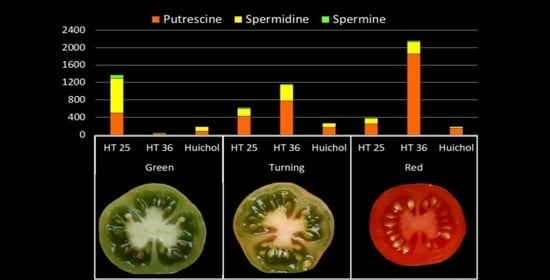
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.C.; Brown, T.R. The Biorenewable Resource Base. In Biorenewable Resources: Engineering New Products from Agriculture; John Wiley & Sons Inc.: Chichester, West Sussex, UK, 2014; p. 388. ISBN 978-1-118-52492-3. [Google Scholar]
- Nadaria-Hoke, S. Structural and Thermodynamic Characterization of Spermidine and Spermine Synthases. Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, 2009. [Google Scholar]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Salvi, D.; Tavladoraki, P. The tree of life of polyamine oxidases. Sci. Rep. 2020, 10, 17858. [Google Scholar] [CrossRef]
- Bae, D.-H.; Lane, D.J.R.; Jansson, P.J.; Richardson, D.R. The old and new biochemistry of polyamines. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Bagni, N.; Tassoni, A. Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 2001, 20, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Tsaniklidis, G.; Kotsiras, A.; Tsafouros, A.; Roussos, P.A.; Aivalakis, G.; Katinakis, P.; Delis, C. Spatial and temporal distribution of genes involved in polyamine metabolism during tomato fruit development. Plant Physiol. Biochem. 2016, 100, 27–36. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Romero, L.; Ruiz, J.M. Accumulation of free polyamines enhances the antioxidant response in fruits of grafted tomato plants under water stress. J. Plant Physiol. 2016, 190, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.M.; Maiale, S.J.; Rossi, F.R.; Marina, M.; Ruíz, O.A.; Gárriz, A. Polyamine metabolism responses to biotic and abiotic stress. In Polyamines; Alcázar, R., Tiburcio, A.F., Eds.; Springer: New York, NY, USA, 2018; Volume 1694, pp. 37–49. ISBN 978-1-4939-7397-2. [Google Scholar]
- Mehta, R.A.; Cassol, T.; Li, N.; Ali, N.; Handa, A.K.; Mattoo, A.K. Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat. Biotechnol. 2002, 20, 613–618. [Google Scholar] [CrossRef]
- Koushesh saba, M.; Arzani, K.; Barzegar, M. Postharvest polyamine application alleviates chilling injury and affects apricot storage ability. J. Agric. Food Chem. 2012, 60, 8947–8953. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Kurihara, S.; Sawaki, E.; Koga, Y.; Benno, Y. Impact of intestinal microbiota on intestinal luminal metabolome. Sci. Rep. 2012, 2, 233. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Kano, Y.; Chiba, F. Food polyamine and cardiovascular disease-An epidemiological study. GJHS 2012, 4, 170. [Google Scholar] [CrossRef]
- Nilsson, B.-O.; Persson, L. Beneficial effects of spermidine on cardiovascular health and longevity suggest a cell type-specific import of polyamines by cardiomyocytes. Biochem. Soc. Trans. 2019, 47, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soda, K.; Dobashi, Y.; Kano, Y.; Tsujinaka, S.; Konishi, F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009, 44, 727–732. [Google Scholar] [CrossRef]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher spermidine intake is linked to lower mortality: A Prospective population-based study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-promoting effects of dietary polyamines. Med. Sci. 2021, 9, 8. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell. Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Miao, H.; Ou, J.; Peng, Y.; Zhang, X.; Chen, Y.; Hao, L.; Xie, G.; Wang, Z.; Pang, X.; Ruan, Z.; et al. Macrophage ABHD5 promotes colorectal cancer growth by suppressing spermidine production by SRM. Nat. Commun. 2016, 7, 11716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Feng, Z.; Chen, N. Spermidine as a target for cancer therapy. Pharmacol. Res. 2020, 159, 104943. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.M.; Gobert, A.P.; Wilson, K.T. The role of polyamines in gastric cancer. Oncogene 2021, 40, 4399–4412. [Google Scholar] [CrossRef]
- Casero, R.A.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.J.; Ashbeck, E.L.; Wertheim, B.C.; Wallace, R.B.; Neuhouser, M.L.; Thomson, C.A.; Thompson, P.A. Dietary polyamine intake and colorectal cancer risk in postmenopausal women. Am. J. Clin. Nutr. 2015, 102, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-Y.; Fang, Y.-J.; Abulimiti, A.; Yang, X.; Li, L.; Liu, K.-Y.; Zhang, X.; Feng, X.-L.; Chen, Y.-M.; Zhang, C.-X. Dietary polyamines intake and risk of colorectal cancer: A case-control study. Nutrients 2020, 12, 3575. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, M.; Groeger, D.; Frei, R.; Ferstl, R.; Wawrzyniak, P.; Krawczyk, K.; Pugin, B.; Barcik, W.; Westermann, P.; Dreher, A.; et al. Spermidine and spermine exert protective effects within the lung. Pharmacol. Res. Perspect. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, metabolism, and role in human disease management. Med. Sci. 2021, 9, 44. [Google Scholar] [CrossRef]
- Simon, P.W. Progress toward increasing intake of dietary nutrients from vegetables and fruits: The case for a greater role for the horticultural sciences. Horts 2014, 49, 112–115. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mier, L.; Jimenez-García, S.N.; Salazar, C.S.; Contreras-Medina, L.M.; Escalante, K.E.; Martinez, C.G.; García-Trejo, J.F.; Guevara-Gonzalez, R.G.; Feregrino-Perez, A.A. Strategies that influence the production of secondary metabolites in plants. In Nutritional Quality Improvement in Plants; Jaiwal, P.K., Chhillar, A.K., Chaudhary, D., Jaiwal, R., Eds.; Concepts and Strategies in Plant Sciences; Springer International Publishing: Cham, Germany, 2019; pp. 231–270. ISBN 978-3-319-95353-3. [Google Scholar]
- Hou, Y.; He, W.; Hu, S.; Wu, G. Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 2019, 51, 1153–1165. [Google Scholar] [CrossRef]
- Takahashi, T. Plant polyamines. Plants 2020, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, S.; Yu, X.; Dong, R.; Li, Y.; Mei, X.; Shen, Y. Polyamines regulate strawberry fruit ripening by abscisic acid, auxin, and ethylene. Plant Physiol. 2018, 177, 339–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortes, A.M.; Agudelo-Romero, P.; Silva, M.S.; Ali, K.; Sousa, L.; Maltese, F.; Choi, Y.H.; Grimplet, J.; Martinez- Zapater, J.M.; Verpoorte, R.; et al. Transcript and metabolite analysis in trincadeira cultivar reveals novel information regarding the dynamics of grape ripening. BMC Plant Biol. 2011, 11, 149. [Google Scholar] [CrossRef] [Green Version]
- Ziosi, V.; Bregoli, A.M.; Bonghi, C.; Fossati, T.; Biondi, S.; Costa, G.; Torrigiani, P. Transcription of ethylene perception and biosynthesis genes is altered by putrescine, spermidine and aminoethoxyvinylglycine (AVG) during ripening in peach fruit (Prunus Persica). New Phytol. 2006, 172, 229–238. [Google Scholar] [CrossRef]
- Yahia, E.M.; Contreras-Padilla, M.; Gonzalez-Aguilar, G. Ascorbic acid content in relation to ascorbic acid oxidase activity and polyamine content in tomato and bell pepper fruits during development, maturation and senescence. LWT Food Sci. Technol. 2001, 34, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Osorio, S.; Alba, R.; Nikoloski, Z.; Kochevenko, A.; Fernie, A.R.; Giovannoni, J.J. Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiol. 2012, 159, 1713–1729. [Google Scholar] [CrossRef] [Green Version]
- Gharbi, E.; Martínez, J.-P.; Benahmed, H.; Fauconnier, M.-L.; Lutts, S.; Quinet, M. Salicylic acid differently impacts ethylene and polyamine synthesis in the glycophyte Solanum Lycopersicum and the wild-related halophyte Solanum Chilense exposed to mild salt stress. Physiol. Plantarum. 2016, 158, 152–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Faostat: Crops-Production Quantity-World-Vegetables Primary. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 26 March 2021).
- Rick, C.M. The Tomato. Sci. Am. 1978, 239, 76–89. [Google Scholar] [CrossRef]
- Altman, P.L.; Dittmer, D.S. Metabolism; Biological handbooks; Federation of American Societies for Experimental Biology: Rockville, MD, USA, 1968. [Google Scholar]
- Gupta, A.; Pandey, R.; Sinha, R.; Chowdhary, A.; Pal, R.; Rajam, M. Improvement of post-harvest fruit characteristics in tomato by fruit-specific over-expression of oat arginine decarboxylase gene. Plant Growth Regul. 2019, 88, 1–11. [Google Scholar] [CrossRef]
- Neily, M.H.; Matsukura, C.; Maucourt, M.; Bernillon, S.; Deborde, C.; Moing, A.; Yin, Y.-G.; Saito, T.; Mori, K.; Asamizu, E.; et al. Enhanced polyamine accumulation alters carotenoid metabolism at the transcriptional level in tomato fruit over-expressing spermidine synthase. J. Plant Physiol. 2011, 168, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Pedro, A.M.K.; Ferreira, M.M.C. Nondestructive determination of solids and carotenoids in tomato products by near-infrared spectroscopy and multivariate calibration. Anal. Chem. 2005, 77, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Akpolat, H.; Barineau, M.; Jackson, K.A.; Aykas, D.P.; Rodriguez-Saona, L.E. Portable infrared sensing technology for phenotyping chemical traits in fresh market tomatoes. LWT 2020, 124, 109164. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Quality and safety of fresh fruits and vegetables at harvest. Sci. Hortic. 2018, 239, 78–79. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture Index of Official Visual Aids. Available online: https://www.ams.usda.gov/sites/default/files/media/Official%20Inventory%20of%20FV%20Inspection%20Aids.pdf (accessed on 16 September 2019).
- Rodríguez, G.R.; Muños, S.; Anderson, C.; Sim, S.-C.; Michel, A.; Causse, M.; Gardener, B.B.M.; Francis, D.; van der Knaap, E. Distribution of SUN, OVATE, LC, and FAS in the tomato germplasm and the relationship to fruit shape diversity. Plant Physiol. 2011, 156, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergquist, S.Å.; Gertsson, U.E.; Olsson, M.E. Influence of growth stage and postharvest storage on ascorbic acid and carotenoid content and visual quality of baby spinach (Spinacia Oleracea, L.). J. Sci. Food Agric. 2006, 86, 346–355. [Google Scholar] [CrossRef]
- López Camelo, A.F.; Gómez, P.A. Comparison of color indexes for tomato ripening. Hortic. Bras. 2004, 22, 534–537. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. Hort. Sci. 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Sun, X.; Lu, H.; Yang, H.; Ruan, Q.; Huang, H.; Chen, M. Detecting and Monitoring the flavor of tomato (Solanum Lycopersicum) under the Impact of postharvest handlings by physicochemical parameters and electronic nose. Sensors 2018, 18, 1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolesa, G.N.; Workneh, T.S.; Melesse, S.F. Logistic regression analysis of marketability of tomato fruit harvested at different maturity stages and subjected to disinfection, storage condition and storage period treatments. Biol. Agric. Hortic. 2018, 34, 40–52. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Hatmi, S.; Gruau, C.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Baillieul, F.; Eullaffroy, P.; Clément, C.; Ferchichi, A.; Aziz, A. Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to botrytis cinerea. J. Exp. Botany 2015, 66, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, A.; Brun, O.; Audran, J.-C. Involvement of polyamines in the control of fruitlet physiological abscission in grapevine (Vitis Vinifera). Physiol. Plant. 2001, 113, 50–58. [Google Scholar] [CrossRef]
- Flores, H.E.; Galston, A.W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982, 69, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eerola, S.; Tiina, R.; Kuitunen, K. Rokka mervi biogenic amines. HPLC determination in foods. NMKL Method 2013, 1, 1–12. [Google Scholar]
- Buranaphalin, S. Pharmaceutical Analysis of Polyamines and Aminoglycosides; University of Bath: Bath, UK, 2009. [Google Scholar]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Fita, A.; García-Martínez, M.D.; Casanova, C.; Borràs, D.; Plazas, M.; Andújar, I.; Soler, S. Characterization of composition traits related to organoleptic and functional quality for the differentiation, selection and enhancement of local varieties of tomato from different cultivar groups. Food Chem. 2015, 187, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Johansson, E.; Hussain, A.; Kuktaite, R.; Andersson, S.C.; Olsson, M.E. Contribution of organically grown crops to human health. Int. J. Environ. Res. Public Health 2014, 11, 3870. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Branlard, G.; Cuniberti, M.; Flagella, Z.; Hüsken, A.; Nurit, E.; Peña, R.J.; Sissons, M.; Vazquez, D. Genotypic and environmental effects on wheat technological and nutritional quality. In Wheat Quality For Improving Processing And Human Health; Igrejas, G., Ikeda, T.M., Guzmán, C., Eds.; Springer International Publishing: Cham, Germnay, 2020; pp. 171–204. ISBN 978-3-030-34162-6. [Google Scholar]
- Vagiri, M.; Ekholm, A.; Öberg, E.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Phenols and ascorbic acid in black currants (Ribes nigrum L.): Variation due to genotype, location and year. J. Agric. Food Chem. 2013, 61, 9298–9306. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.C.; Olsson, M.E.; Gustavsson, K.-E.; Johansson, E.; Rumpunen, K. Tocopherols in rose hips (Rosa Spp.) during Ripening. J. Sci. Food Agric. 2012, 92, 2116–2121. [Google Scholar] [CrossRef]
- Johansson, E.; Kuktaite, R.; Andersson, A.; Prieto-Linde, M.L. Protein polymer build-up during wheat grain development: Influences of temperature and nitrogen timing. J. Sci. Food Agric. 2005, 85, 473–479. [Google Scholar] [CrossRef]
- Andersson, S.C.; Olsson, M.E.; Johansson, E.; Rumpunen, K. Carotenoids in sea buckthorn (Hippophae Rhamnoides, L.) berries during ripening and use of pheophytin a as a maturity marker. J. Agric. Food Chem. 2009, 57, 250–258. [Google Scholar] [CrossRef]
- Arias, R.; Lee, T.-C.; Logendra, L.; Janes, H. Correlation of lycopene measured by HPLC with the L *, a *, b * color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. J. Agric. Food Chem. 2000, 48, 1697–1702. [Google Scholar] [CrossRef]
- Opara, U.L.; Al-Ani, M.R.; Al-Rahbi, N.M. Effect of fruit ripening stage on physico-chemical properties, nutritional composition and antioxidant components of tomato (Lycopersicum Esculentum) cultivars. Food Bioprocess Technol. 2012, 5, 3236–3243. [Google Scholar] [CrossRef]
- Clément, A.; Dorais, M.; Vernon, M. Multivariate approach to the measurement of tomato maturity and gustatory attributes and their rapid assessment by vis−NIR spectroscopy. J. Agric. Food Chem. 2008, 56, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.H.; McGlasson, B.; Joyce, D.; Graham, D. Postharvest: An Introduction to the Physiology and Handling of Fruit, Vegetables and Ornamentals; University of Texas Press: Austin, TX, USA, 2007; ISBN 978-1-84593-227-5. [Google Scholar]
- Zsom-Muha, V.; Zsom, T.; Felföldi, J. In-vivo measurement of tomato firmness. Acta Hortic. 2008, 677–684. [Google Scholar] [CrossRef]
- Nambeesan, S.; Datsenka, T.; Ferruzzi, M.G.; Malladi, A.; Mattoo, A.K.; Handa, A.K. Overexpression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato: Polyamines enhance shelf life in tomato. Plant J. 2010, 63, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Madhulatha, P.; Gupta, A.; Gupta, S.; Kumar, A.; Pal, R.K.; Rajam, M.V. Fruit-specific over-expression of human s-adenosylmethionine decarboxylase gene results in polyamine accumulation and affects diverse aspects of tomato fruit development and quality. J. Plant Biochem. Biotechnol. 2014, 23, 151–160. [Google Scholar] [CrossRef]
- Anwar, R.; Fatima, S.; Mattoo, A.K.; Handa, A.K. Fruit architecture in polyamine-rich tomato germplasm is determined via a medley of cell cycle, cell expansion, and fruit shape genes. Plants 2019, 8, 387. [Google Scholar] [CrossRef] [Green Version]
- Goyal, R.K.; Fatima, T.; Topuz, M.; Bernadec, A.; Sicher, R.; Handa, A.K.; Mattoo, A.K. Pathogenesis-related protein 1b1 (PR1b1) is a major tomato fruit protein responsive to chilling temperature and upregulated in high polyamine transgenic genotypes. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Nishibori, N.; Fujihara, S.; Akatuki, T. Amounts of polyamines in foods in Japan and Intake by Japanese. Food Chem. 2007, 100, 491–497. [Google Scholar] [CrossRef]
- Moret, S.; Smela, D.; Populin, T.; Conte, L.S. A Survey on free biogenic amine content of fresh and preserved vegetables. Food Chem. 2005, 89, 355–361. [Google Scholar] [CrossRef]
- Harpaz-Saad, S.; Yoon, G.M.; Mattoo, A.K.; Kieber, J.J. The Formation of ACC and competition between polyamines and ethylene for SAM. In Annual Plant Reviews Volume 44; McManus, M.T., Ed.; Wiley-Blackwell: Oxford, UK, 2012; pp. 53–81. ISBN 978-1-118-22308-6. [Google Scholar]
- Ben-arie, R.; Lurie, S.; Mattoo, A. Temperature-dependent inhibitory effects of calcium and spermine on ethylene biosynthesis in apple discs correlate with changes in microsomal membrane microviscosit. Plant Sci. Lett. 1982, 24, 239–247. [Google Scholar] [CrossRef]
- Apelbaum, A.; Burgoon, A.C.; Anderson, J.D.; Lieberman, M.; Ben-Arie, R.; Mattoo, A.K. Polyamines inhibit biosynthesis of ethylene in higher plant tissue and fruit protoplasts. Plant Physiol. 1981, 68, 453–456. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Mei, X.; Li, Y.; Guo, J.; Shen, Y. Update on the roles of polyamines in fleshy fruit ripening, senescence, and quality. Front. Plant Sci. 2021, 12, 610313. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Minocha, S.C.; Minocha, R.; Handa, A.K. Polyamines and cellular metabolism in plants: Transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids 2010, 38, 405–413. [Google Scholar] [CrossRef]
- Li, N.; Parsons, B.L.; Liu, D.; Mattoo, A.K. Accumulation of wound-inducible ACC synthase transcript in tomato fruit is inhibited by salicylic acid and polyamines. Plant Mol. Biol. 1992, 18, 477–487. [Google Scholar] [CrossRef]
- Zhang, T.; John, S.; Wang, Y. Cultivar and Agricultural Management on Lycopene and Vitamin C Contents in Tomato Fruits; CRC Press: Boca Raton, FL, USA, 2008; pp. 27–45. [Google Scholar]
- Gómez, M.D.; Vera-Sirera, F.; Pérez-Amador, M.A. Molecular programme of senescence in dry and fleshy fruits. J. Exp. Botany 2013, 65, 4515–4526. [Google Scholar] [CrossRef] [Green Version]
- Morilla, A.; García, J.M.; Albi, M.A. Free polyamine contents and decarboxylase activities during tomato development and ripening. J. Agric. Food Chem. 1996, 44, 2608–2611. [Google Scholar] [CrossRef]
- Wi, S.J.; Kim, W.T.; Park, K.Y. Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep. 2006, 25, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Kasukabe, Y.; He, L.; Nada, K.; Misawa, S.; Ihara, I.; Tachibana, S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis Thaliana. Plant Cell Physiol. 2004, 45, 712–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasukabe, Y.; He, L.; Watakabe, Y.; Otani, M.; Shimada, T.; Tachibana, S. Improvement of environmental stress tolerance of sweet potato by introduction of genes for spermidine synthase. Plant Biotechnol. 2006, 23, 75–83. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Ban, Y.; Inoue, H.; Matsuda, N.; Liu, J.; Moriguchi, T. Enhancement of spermidine content and antioxidant capacity in transgenic pear shoots overexpressing apple spermidine synthase in response to salinity and hyperosmosis. Phytochemistry 2008, 69, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-P.; Pang, X.-M.; Matsuda, N.; Kita, M.; Inoue, H.; Hao, Y.-J.; Honda, C.; Moriguchi, T. Over-expression of the apple spermidine synthase gene in pear confers multiple abiotic stress tolerance by altering polyamine titers. Transgenic Res. 2008, 17, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Fortes, A.M.; Agudelo-Romero, P. Polyamine metabolism in climacteric and non-climacteric fruit ripening. In Polyamines; Alcázar, R., Tiburcio, A.F., Eds.; Springer: New York, NY, USA, 2018; Volume 1694, pp. 433–447. ISBN 978-1-4939-7397-2. [Google Scholar]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Tan, B.; Ren, W.; Rahu, N.; Dad, R.; Kalhoro, D.H.; Yin, Y. Polyamines: Therapeutic perspectives in oxidative stress and inflammatory diseases. Amino Acids 2017, 49, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Saiki, S.; Sasazawa, Y.; Fujimaki, M.; Kamagata, K.; Kaga, N.; Taka, H.; Li, Y.; Souma, S.; Hatano, T.; Imamichi, Y.; et al. A metabolic profile of polyamines in parkinson disease: A promising biomarker. Ann. Neurol. 2019, 86, 251–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Fleete, M.S.; Jing, Y.; Collie, N.D.; Curtis, M.A.; Waldvogel, H.J.; Faull, R.L.M.; Abraham, W.C.; Zhang, H. Altered arginine metabolism in Alzheimer’s disease brains. Neurobiol. Aging 2014, 35, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, E.E.V.; Johansson, E.; Centellas Quezada, A.; Gustavsson, K.-E.; Olsson, M.E. Genotype and Maturity Stage Affect the Content and Composition of Polyamines in Tomato—Possible Relations to Plant and Human Health. Horticulturae 2021, 7, 300. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae7090300
Gutierrez EEV, Johansson E, Centellas Quezada A, Gustavsson K-E, Olsson ME. Genotype and Maturity Stage Affect the Content and Composition of Polyamines in Tomato—Possible Relations to Plant and Human Health. Horticulturae. 2021; 7(9):300. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae7090300
Chicago/Turabian StyleGutierrez, Evelyn E. Villanueva, Eva Johansson, Alberto Centellas Quezada, Karl-Erik Gustavsson, and Marie E. Olsson. 2021. "Genotype and Maturity Stage Affect the Content and Composition of Polyamines in Tomato—Possible Relations to Plant and Human Health" Horticulturae 7, no. 9: 300. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae7090300