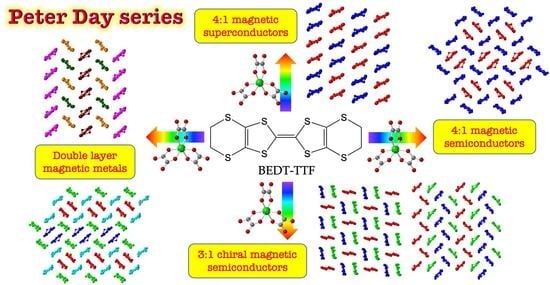
The Peter Day Series of Magnetic (Super)Conductors †
Abstract
:1. Introduction
2. The Superconducting Monoclinic β″ Phase
2.1. β″-BEDT-TTF Salts with the [Fe(C2O4)3]3− Anion
| # | CCDC | Formula a | SG b | Elect. Prop | A+ | G | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | BEMPEO | (ET)4[(H3O)Fe(ox)3]·0.5 py | C2/c | M > 116 K | H3O+ | Py | [15] |
| 2 | BEMQAL | (ET)4[(H3O)Fe(ox)3]·py | C2/c | - | H3O+ | py | [15] |
| 3 | COQNEB | (ET)4[(H2O)Fe(ox)3]·PhNO2 | C2/c | σ = 10 S/cm Semi | - | PhNO2 | [22] |
| 4 | ECOPIV | (ET)4[(H3O/NH4)Fe(ox)3]·PhNO2 | C2/c | Tc = 6.2 K | H3O+/NH4+ | PhNO2 | [14] |
| 5 | KILFOB | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.78(py)0.22 | C2/c | Tc = 3.9 K | H3O+ | PhCN/py | [16] |
| 6 | KILFOB01 | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.77(py)0.23 | C2/c | - | H3O+ | PhCN/py | [16] |
| 7 | KILFOB02 | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.66(py)0.34 | C2/c | Tc = 5.8 K | H3O+ | PhCN/py | [16] |
| 8 | KILFOB03 | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.62(py)0.38 | C2/c | Tc = 6.9 K | H3O+ | PhCN/py | [16] |
| 9 | KILFOB04 | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.57(py)0.43 | C2/c | Tc = 6.7 K | H3O+ | PhCN/py | [16] |
| 10 | KILGOC | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.46(py)0.54 | C2/c | Tc = 5.9 K | H3O+ | PhCN/py | [16] |
| 11 | KILGUI | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.39(py)0.61 | C2/c | Tc = 4.2 K | H3O+ | PhCN/py | [16] |
| 12 | KILHAP | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.10(py)0.90 | C2/c | Tc = 7.3 K | H3O+ | PhCN/py | [16] |
| 13 | PONMEL | (ET)4[(H3O)Fe(ox)3]·1,2-PhCl2 | C2/c | M > 0.5 K | H3O+ | 1,2-PhCl2 | [23] |
| 14 | QAXSIT | (ET)4[(H3O)Fe(ox)3]·PhI | C2/c | σ = 3.4 S/cm Ea = 64 meV | H3O+ | PhI | [20] |
| 15 | SAPWEM | (ET)4[(H3O)Fe(ox)3]·PhBr | C2/c | Tc = 4.0 K | H3O+ | PhBr | [21] |
| 16 | SAPWEM02 | (ET)4[(H3O)Fe(ox)3]·PhBr | P-1 | Tc = 4.0 K | H3O+ | PhBr | [20] |
| 17 | UJOXAT | (ET)4[(H3O)Fe(ox)3]·PhCl | C2/c | M > 0.4 K | H3O+ | PhCl | [17,20] |
| 18 | UJOXAT01 | (ET)4[(H3O)Fe(ox)3]·PhCl | P-1 | M > 4.2 K | H3O+ | PhCl | [18,20] |
| 19 | UJOXEX | (ET)4[(H3O)Fe(ox)3]·PhF | C2/c | Tc = 1.0 K | H3O+ | PhF | [17,20] |
| 20 | UJOXEX01 | (ET)4[(H3O)Fe(ox)3]·PhF | P-1 | Tc = 1.0 K | H3O+ | PhF | [18,20] |
| 21 | UJOXIB | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.4(PhF)0.6 | C2/c | Tc = 6.0 K | H3O+ | PhCN/PhF | [17] |
| 22 | UJOXIB01 | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.4(PhF)0.6 | P-1 | Tc = 6.0 K | H3O+ | PhCN/PhF | [18] |
| 23 | UJOXOH | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.86(PhCl2)0.14 | C2/c | Tc = 7.2 K | H3O+ | PhCN/PhCl2 | [17] |
| 24 | UJOYAU | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.35(PhCl)0.65 | C2/c | Tc = 6.0 K | H3O+ | PhCN/PhCl | [17] |
| 25 | UJOYAU02 | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.35(PhCl)0.65 | P-1 | Tc = 6.0 K | H3O+ | PhCN/PhCl | [18] |
| 26 | UJOYEY | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.17(PhBr)0.83 | C2/c | Tc = 4.2 K | H3O+ | PhCN/PhBr | [17] |
| 27 | UJOYEY01 | (ET)4[(H3O)Fe(ox)3]·(PhCN)0.17(PhBr)0.83 | P-1 | Tc = 4.2 K | H3O+ | PhCN/PhBr | [18] |
| 28 | UMACEQ | (ET)4[(NH4)Fe(ox)3]·dmf | C2/c | M > 4.2 K | NH4+ | dmf | [13] |
| 29 | YUYTUJ | (ET)4[(H3O)Fe(ox)3]·2-Clpy | C2/c | Tc = 2.4–4.0 K | H3O+ | 2-Clpy | [19] |
| 30 | YUYTUJ01 | (ET)4[(H3O)Fe(ox)3]·2-Clpy | P-1 | Tc = 2.4–4.0 K | H3O+ | 2-Clpy | [19] |
| 31 | YUYVEV | (ET)4[(H3O)Fe(ox)3]·2-Brpy | C2/c | Tc = 4.3 K | H3O+ | 2-Brpy | [19] |
| 32 | YUYVEV01 | (ET)4[(H3O)Fe(ox)3]·2-Brpy | P-1 | Tc = 4.3 K | H3O+ | 2-Brpy | [19] |
| 33 | YUYVOF | (ET)4[(H3O)Fe(ox)3]·3-Clpy | C2/c | M > 0.5 K | H3O+ | 3-Clpy | [19] |
| 34 | YUYVUL | (ET)4[(H3O)Fe(ox)3]·3-Brpy | C2/c | M > 0.5 K | H3O+ | 3-Brpy | [19] |
| 35 | ZIGYET | (ET)4[(H3O)Fe(ox)3]·PhCN | C2/c | Tc = 6.5–8.5 K | H3O+ | PhCN | [2,3,4,17] |
2.2. β″-BEDT-TTF Salts with Other [M(C2O4)3]3− Anions (M ≠ Fe)
| # | CCDC | Formula a | SG b | Elect. Prop. | A+ | M | G | Ref. |
|---|---|---|---|---|---|---|---|---|
| 36 | CIWMED | (ET)4[K0.8(H3O)0.2Cr(ox)3]·2-Clpy | C2/c | TMI ≈ 10 K | K+/H3O+ | Cr | 2-Clpy | [27] |
| 37 | CIWMIH | (ET)4[K0.8(H3O)0.2Cr(ox)3]·2-Brpy | C2/c | TMI ≈ 10 K | K+/H3O+ | Cr | 2-Brpy | [27] |
| 38 | CIWMON | (ET)4[K0.8(H3O)0.2Ga(ox)3]·2-Clpy | C2/c | TMI ≈ 10 K | K+/H3O+ | Ga | 2-Clpy | [27] |
| 39 | CIWMUT | (ET)4[K0.8(H3O)0.2Ga(ox)3]·2-Brpy | C2/c | TMI ≈ 10 K | K+/H3O+ | Ga | 2-Brpy | [27] |
| 40 | ECOPUH | (ET)4[(H3O/NH4)Cr(ox)3]·PhNO2 | C2/c | Tc = 5.8 K | H3O+/NH4+ | Cr | PhNO2 | [14] |
| 41 | FEBLIK | (ET)4[(NH4)Rh(ox)3]·PhBr | C2/c | Tc = 2.5 K | NH4+ | Rh | PhBr | [32] |
| 42 | FECDAV | (ET)4[(H3O)Rh(ox)3]·PhF | C2/c | TMI ≈ 180 K | H3O+ | Rh | PhF | [32] |
| 43 | FECDID | (ET)4[(NH4)Rh(ox)3]·PhCl | C2/c | TMI ≈ 10 K | NH4+ | Rh | PhCl | [32] |
| 44 | HOBROH | (ET)4[K0.33(H3O)0.67Ga(ox)3]·PhBr | C2/c | Semi | K+/H3O+ | Ga | PhBr | [30] |
| 45 | HUNQIQ | (ET)4[(H3O)Ga(ox)3]·py | C2/c | Tc ≈ 2 K ?? | H3O+ | Ga | py | [29] |
| 46 | HUNQUC | (ET)4[(H3O)Ga(ox)3]·PhNO2 | C2/c | Tc = 7.5 K | H3O+ | Ga | PhNO2 | [29] |
| 47 | JUPGUW01 | (ET)4[(H3O)Cr(ox)3]·PhCN | C2/c | Tc = 6.0 K | H3O+ | Cr | PhCN | [4,24,25] |
| 48 | MEQZIR | (ET)4[(H3O)Cr(ox)3]·CH2Cl2 | C2/c | Semi | H3O+ | Cr | CH2Cl2 | [28] |
| 49 | UDETUU | (ET)4[K0.7(H3O)0.3Ru(ox)3]·PhBr | C2/c | Tc = 2.8–6.3 K | K+/H3O+ | Ru | PhBr | [31] |
| 50 | UMACAM | (ET)4[(K/NH4)Cr(ox)3]·dmf | C2/c | M > 4.2 | K+/NH4+ | Cr | dmf | [13] |
| 51 | UMACIU | (ET)4[KCr(ox)3]·dmf | C2/c | M > 4.2 | K+ | Cr | dmf | [13] |
| 52 | - | (ET)4[(H3O)Mn(ox)3]·PhBr | C2/c | Tc = 2.0 K | H3O+ | Mn | PhBr | [20] |
| 53 | - | (ET)4[(H3O)Cr(ox)3]·PhBr | C2/c | Tc = 1.7 K | H3O+ | Cr | PhBr | [21,26] |
| 54 | - | (ET)4[(H3O)Cr(ox)3]·PhCl | C2/c | M > 130 K σ300 = 3 × 10−3 S/cm | H3O+ | Cr | PhCl | [26] |
3. The Semiconducting pseudo-κ or κ′ Phase
κ′-BEDT-TTF Radical Salts with [M(C2O4)3]3− Anions
| # | CCDC | Formula a | SG b | Elect. Prop. | A+ | M | G | Ref. |
|---|---|---|---|---|---|---|---|---|
| 55 | CISMEZ | κ′-(ET)4[KMn(ox)3]·PhCN | Pbcn | σ = 2 × 10−5 S/cm Ea = 180 meV | K+ | Mn | PhCN | [33] |
| 56 | FECDEZ | κ′-(ET)4[(NH4)Rh(ox)3]·PhCN | Pbcn | Ea = 245 meV | NH4+ | Rh | PhCN | [32] |
| 57 | JUPGUW | κ′-(ET)4[(H3O)Cr(ox)3]·PhCN | Pbcn | Ea = 153 meV | H3O+ | Cr | PhCN | [4,25] |
| 58 | QIWMOY | κ′-(ET)4[(NH4)Co(ox)3]·PhCN | Pbcn | Ea = 225 meV | NH4+ | Co | PhCN | [4] |
| 59 | QIWMUE | κ′-(ET)4[(NH4)Al(ox)3]·PhCN | Pbcn | Ea = 222 meV | NH4+ | Al | PhCN | [4] |
| 60 | UDETOO | κ′-(ET)4[K0.8(H3O)0.2Ru(ox)3]·PhCN | Pbcn | Semi | K+/H3O+ | Ru | PhCN | [31] |
| 61 | UJOXUN | κ′-(ET)4[(H3O)Fe(ox)3]·(PhCN)0.88(PhCl2)0.12 | Pbcn | Semi | H3O+ | Fe | PhCl2/PhCN | [17] |
| 62 | ZIWNEY | κ′-(ET)4[(NH4)Fe(ox)3]·PhCN | Pbcn | σ = 10−4 S/cm Ea = 140 meV | NH4+ | Fe | PhCN | [2,4] |
| 63 | ZIWNIC | κ′-(ET)4[KFe(ox)3]·PhCN | Pbcn | σ = 10−4 S/cm Ea = 140 meV | K+ | Fe | PhCN | [2] |
4. Other Phases with BEDT-TTF and Oxalato Complexes
4.1. BEDT-TTF Salts with [M(C2O4)3]3− Anions and 18-Crown-6
| # | CCDC | Formula a | SG b | Elect. Prop. | A+ | M | G | Ref. |
|---|---|---|---|---|---|---|---|---|
| 64 | ACAGUG | β″-(ET)4[(H3O)Cr(ox)3]2[(H3O)2(18-c-6)]·5H2O | P-1 | σ = 300 S/cm TMI = 190 K | H3O+ | Cr | H2O/18-c-6 | [34,35] |
| 65 | COLWUY | β″-(ET)2[(H2O)(NH4)2Ir(ox)3]·18-c-6 | P-1 | TMI ≈ 100 K | NH4+ | Ir | H2O/18-c-6 | [36] |
| 66 | COLYOU | β″-(ET)2[(H2O)(NH4)2Ru(ox)3]·18-c-6 | P-1 | TMI ≈ 155 K | NH4+ | Ru | H2O/18-c-6 | [36] |
| 67 | FENHEO | β″-(ET)2[(H2O)(NH4)2Cr(ox)3]·18-c-6 | P-1 | Tc = 4.0–4.9 K | NH4+ | Cr | H2O/18-c-6 | [37] |
| 68 | FEQQAU | β″-(ET)4[(NH4)Ga(ox)3]2[(NH4)2(18-c-6)]·5H2O | P-1 | σ = 200 S/cm TMI = 240 K | NH4+ | Ga | H2O/18-c-6 | [35] |
| 69 | KATLAV | β″-(ET)2[(H2O)(NH4)2Rh(ox)3]·18-c-6 | P-1 | Tc = 2.7 K | NH4+ | Rh | H2O/18-c-6 | [38] |
| 70 | NIHPEA | α-(ET)10(18-c-6)6K6[Fe(ox)3]4·24H2O | P21/c | Ea = 105 meV | K+ | Fe | H2O/18-c-6 | [39] |
| 71 | UJEYIR | (ET)4[Ga(ox)3](18-c-6)(H2O)6 | P-1 | - | - | Ga | H2O/18-c-6 | c |
4.2. BEDT-TTF Salts with [M(C2O4)3]3− Anions and Two Different Donor Layers

| # | CCDC | Formula a | SG b | Elect. Prop. | A+ | M | G | Ref. |
|---|---|---|---|---|---|---|---|---|
| 72 | AQUZUH | α,β″-(ET)4[(NH4)Ga(ox)3] ·PhN(CH3)CHO | P-1 | σ = 0.26–0.60 S/cm | NH4+ | Ga | PhN(CH3)CHO | [40] |
| 73 | ARABAW | α,β″-(ET)4[(NH4)Ga(ox)3] ·PhCH2CN | P-1 | σ = 0.24–1.34 S/cm | NH4+ | Ga | PhCH2CN | [40] |
| 74 | ARABEA | α,β″-(ET)4[(NH4)Fe(ox)3]·PhCOCH3 | P-1 | - | NH4+ | Fe | PhCOCH3 | [40] |
| 75 | CILDIL | α,β″-(ET)4[(NH4)Fe(ox)3] ·(S)-PhC(OH)HCH3 | P1 | σ = 5.3 S/cm TMI = 170 K | NH4+ | Fe | (S)-PhC(OH)OCH3 | [41] |
| 76 | HOBRIB | α,κ′-(ET)4K0.45(H3O)0.55[Ga(ox)3] ·1,2-PhBr2 | P-1 | Metal | K+/H3O+ | Ga | 1,2-PhBr2 | [30] |
| 77 | NIPTEM | α,β″-(ET)4[(NH4)Fe(ox)3] ·(R/S)-PhC(OH)HCH3 | P-1 | σ = 10.4 S/cm TMI = 150 K | NH4+ | Fe | (R/S)-PhC(OH)HCH3 | [41] |
| 78 | TANDIX | α,κ′-(ET)4[(H3O)Fe(ox)3]·1,2-PhBr2 | P-1 | M > 1.5 K | H3O+ | Fe | PhBr2 | [42] |
4.3. BEDT-TTF:[M(C2O4)3]3− Phases with 3:1 Stoichiometry
| # | CCDC | Formula a | SG b | Elect. Prop. | A+ | M | G | Ref. |
|---|---|---|---|---|---|---|---|---|
| 79 | BOYTIU | (ET)3[NaAl(ox)3]·CH3NO2 | P21 | Ea = 140 meV | Na+ | Al | CH3NO2 | [43] |
| 80 | BOYTOA | (ET)3[(NH4)0.83Cr1.17(ox)3]·CH3NO2 | P212121 | Ea = 140 meV | Na+ | Cr | CH3NO2 | [43] |
| 81 | DUDWUW | (ET)3[LiCr(ox)3]·EtOH | P21/c | Ea = 179 meV | Li+ | Cr | EtOH | [46] |
| 82 | DUDXAD | (ET)3[LiFe(ox)3]·EtOH | P21/c | Ea = 126 meV | Li+ | Fe | EtOH | [46] |
| 83 | DUXNOA | (ET)3[Na(Δ-Cr(ox)3)0.56(Λ-Cr(ox)3)0.44]·CH2Cl2 | P1 | Ea = 69 meV | Na+ | Cr | CH2Cl2 | [47] |
| 84 | KOGMUQ01 | (ET)3[NaCr(ox)3]·CH3CN | P21 | σ = 0.038 S/cmEa = 172 meV | Na+ | Cr | CH3CN | [44] |
| 85 | XUNXOU | (ET)3{Na[⊗-Cr(ox)3]0.64[Λ-Cr(ox)3]0.36}·CH3NO2 | P212121 | σ = 0.5 S/cm Ea = 80 meV | Na+ | Cr | CH3NO2 | [45] |
| 86 | XUNXOU01 | (ET)3[NaCr(ox)3]·CH3NO2 | P21 | σ = 0.045 S/cm Ea = 79 meV | Na+ | Cr | CH3NO2 | [45] |
| 87 | YUCLOZ | (ET)3[NaCr(ox)3]·EtOH | P1 | Semi | Na+ | Cr | EtOH | [44] |
| 88 | YUCLUF | θ-(ET)3[NaCr(ox)3]·dmf | P1 | Semi | Na+ | Cr | dmf | [44] |
4.4. Other Phases of BEDT-TTF Salts with [M(C2O4)3]3− Anions
| # | CCDC | Formula a | SG b | Elect. Prop. | A+ | M | G | Ref. |
|---|---|---|---|---|---|---|---|---|
| 89 | CIWNAA | α″-(ET)5[Ga(ox)3]·3.4H2O·0.6EtOH | Pbca | Ea = 71 meV | - | Ga | EtOH/H2O | [27] |
| 90 | DUDWOQ | η-(ET)4[(H2O)LiFe(ox)3] | P21/c | σ = 0.41 S/cm Ea = 80 meV | Li+/H2O | Fe | - | [46] |
| 91 | DUXNUG | α‴-(ET)9Na18[Cr(ox)3]8·24H2O | P-1 | Ea = 66 meV | Na+ | Cr | H2O | [47] |
| 92 | IPEKAQ | α-(ET)6[Fe(ox)3] | P21 | - | - | Fe | H2O/EtOH ? | [17] |
| 93 | KIVKAC | α-(ET)12[Fe(ox)3]2·15H2O | C2/c | σ = 0.055 S/cm | H3O+ | Fe | H2O | [51] |
| 94 | KIVKEG | α-(ET)12[Fe(ox)3]2·16H2O | C2/c | σ = 0.111 S/cm | H3O+ | Fe | H2O | [51] |
| 95 | NIHPAW | α‴-(ET)9Na18[Fe(ox)3]8·24H2O | P-1 | Ea = 77 meV | Na+ | Fe | H2O | [39] |
| 96 | OGUPAI | β″-(ET)5[Fe(ox)3](H2O)2CH2Cl2 | P-1 | σ = 4 S/cm Ea = 30 meV | - | Fe | CH2Cl2/H2O | [52] |
4.5. BEDT-TTF Salts with [Ge(C2O4)3]2− and [Cu(C2O4)2]2− Dianions

| # | CCDC | Formula a | SG b | Elect. Prop. | G | Anion | Ref. |
|---|---|---|---|---|---|---|---|
| 97 | MAJYUR | (ET)2[Ge(ox)3]·PhCN | P21/c | Ea = 127 meV | PhCN | [Ge(ox)3]2− | [53] |
| 98 | MUVFUF | (ET)5[Ge(ox)3]2 | C2 | σ = 10−3 S/cm Ea = 225 meV | - | [Ge(ox)3]2− | [54] |
| 99 | MUVGAM | (ET)7[Ge(ox)3]2·0.87CH2Cl2·0.09H2O | C2/c | σ = 1.75 S/cm Ea = 117–172 meV | CH2Cl2/H2O | [Ge(ox)3]2− | [54] |
| 100 | PADDOQ | (ET)4[Ge(ox)3].0.5CH2Cl2 | P21/c | σ = 4.7 × 10−3 S/cm Ea = 224 meV | CH2Cl2 | [Ge(ox)3]2− | [55] |
| 101 | SOJLUY | (ET)4[Cu(ox)2] | P-1 | TM-I = 65 K Ea = 15 meV | - | [Cu(ox)2]2− | [56,57] |
5. BEDT-TTF Salts with Oxalate Dimers and 2D Lattices
| # | CCDC | Formula a | SG b | Elect. Prop. | Anion | G | Ref. |
|---|---|---|---|---|---|---|---|
| 102 | CEMMUF | (ET)3[Cu2(ox)3]·CH2Cl2 | P-1 | - | [Cu2(ox)3]2− | CH2Cl2 | c |
| 103 | IPOZIY | (ET)2.53[MnCr(ox)3]·CH2Cl2 | P-1 | σ = 10 S/cm metal > 0.4 K | [MnCr(ox)3]− | CH2Cl2 | [8] |
| 104 | IPOZOE | (ET)2.53[MnRh(ox)3]·CH2Cl2 | P-1 | σ = 13 S/cm metal > 100 K | [MnRh(ox)3]− | CH2Cl2 | [8] |
| 105 | LOHWIO | (ET)4[Fe2(ox)5] | P21/n | σ = 2 × 10−3 S/cm Ea = 1200 meV | [Fe2(ox)5]4− | - | [58] |
| 106 | NALVIG | (ET)3[MnCr(ox)3] | P-1 | σ = 250 S/cm metal > 0.3 K | [MnCr(ox)3]2− | - | [7] |
| 107 | SAMMEA | (ET)3[Cu2(ox)3]·2CH3OH | P-1 | σ = 4 S/cm Ea = 50 meV | [Cu2(ox)3]2− | CH3OH | [62] |
| 108 | WUXWET | (ET)3[Cu2(ox)3]·2H2O | P-1 | - | [Cu2(ox)3]2− | H2O | c |
6. Radical Salts of Metal-Oxalate Anions with Other TTF-Type Donor Molecules
6.1. TTF and TM-TTF Salts with Oxalate Complexes
6.2. Salts with Se-Containing Donors (BEST and BETS)
6.3. Salts with Other Donors
| # | CCDC | Formula a | SG b | Elect. Prop. | Donor c | G | Ref |
|---|---|---|---|---|---|---|---|
| 109 | CEWMEX | (BEST)4[Cr(ox)3]·PhCOOH·H2O | P-1 | σ = 1.5 S/cm Ea = 49 meV | BEST | PhCOOHH2O | [68] |
| 110 | CEWMIB | (BEST)4[Fe(ox)3]·PhCOOH·H2O | P-1 | σ = 6.4 S/cm Ea = 54 meV | BEST | PhCOOHH2O | [68] |
| 111 | CEWMOH | (BEST)4[Cr(ox)3]·1.5H2O | C2/m | σ = 8.5 S/cm Ea = 62 meV | BEST | H2O | [68] |
| 112 | CEWMUN | (BEST)9[Fe(ox)3]2·7H2O | P-1 | σ = 2.4 S/cm Ea = 44 meV | BEST | H2O | [68] |
| 113 | CEWNAU | (BEST)4[Fe(ox)3]·1.5H2O | C2/m | σ = 14.0 S/cm Ea = 60 meV | BEST | H2O | [68] |
| 114 | DIQFOY | (TTF)7[Fe(ox)3]2·4H2O | P21/c | σ = 10−4 S/cm Ea = 279 meV | TTF | H2O | [59,60] |
| 115 | DIQFUE | (TTF)5[Fe2(ox)5]·2PhCH3·2H2O | C2/m | σ = 1.8 × 10−6 S/cm | TTF | PhCH3H2O | [59,60] |
| 116 | DIQGAL | (TMTTF)4[Fe2(ox)5]·PhCN·4H2O | P-1 | σ = 2.2 × 10−3 S/cm Ea = 290 meV | TMTTF | PhCNH2O | [59,60] |
| 117 | NIDDIP | (BETS)3[Cu2(ox)3]·2CH3OH | P-1 | M > 180 K | BETS | CH3OH | [69] |
| 118 | OLABAE | (TTF)3[Ru(ox)3]·0.5EtOH·4H2O | C2/c | σ = 1.5 × 10−4 S/cm Ea = 61 meV | TTF | EtOHH2O | [64] |
| 119 | RUDNOT02 | (BETS)3[MnCr(ox)3]·CH2Cl2 | P-1 | M > 150 K | BETS | CH2Cl2 | [70] |
| 120 | TUHDOP | (TTF)4{Mn(H2O)2[Cr(ox)3]2}·14H2O | C2/c | σ = 2 × 10−4 S/cm Ea = 200 meV | TTF | H2O | [65] [66] |
| 121 | VIPYUQ | (DMPET)4[Fe2(ox)5] | P21 | - | DMPET | - | [71] |
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robin, M.B.; Day, P. Mixed Valence Chemistry-A Survey and Classification. Adv. Inorg. Chem. Radiochem. 1968, 10, 247–422. [Google Scholar]
- Kurmoo, M.; Graham, A.W.; Day, P.; Coles, S.J.; Hursthouse, M.B.; Caulfield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W. Superconducting and Semiconducting Magnetic Charge Transfer Salts: (BEDT-TTF)4AFe(C2O4)3·C6H5CN (A = H2O, K, NH4). J. Am. Chem. Soc. 1995, 117, 12209–12217. [Google Scholar] [CrossRef]
- Graham, A.W.; Kurmoo, M.; Day, P. β″-(BEDT-TTF)4[(H2O)Fe(C2O4)3]·PhCN: The First Molecular Superconductor Containing Paramagnetic Metal Ions. J. Chem. Soc. Chem. Commun. 1995, 2061–2062. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Guionneau, P.; Howard, J.A.K.; Hibbs, D.E.; Light, M.E.; Hursthouse, M.B.; Uruichi, M.; Yakushi, K. Crystal Chemistry and Physical Properties of Superconducting and Semiconducting Charge Transfer Salts of the Type (BEDT-TTF)4[AIMIII(C2O4)3]·PhCN (AI = H3O, NH4, K.; MIII = Cr, Fe, Co, Al; BEDT-TTF = Bis(Ethylenedithio)Tetrathiafulvalene). Inorg. Chem. 2001, 40, 1363–1371. [Google Scholar] [CrossRef]
- Ojima, E.; Fujiwara, H.; Kato, K.; Kobayashi, H.; Tanaka, H.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. Antiferromagnetic Organic Metal Exhibiting Superconducting Transition, κ-(BETS)2FeBr4 [BETS = Bis(Ethylenedithio)Tetraselenafulvalene]. J. Am. Chem. Soc. 1999, 121, 5581–5582. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fujiwara, E.; Fujiwara, H.; Tanaka, H.; Otsuka, T.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. Antiferromagnetic Organic Superconductors, BETS2FeX4 (X = Br, Cl). Mol. Cryst. Liq. Cryst. 2002, 380, 139–144. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Laukhin, V. Coexistence of Ferromagnetism and Metallic Conductivity in a Molecule-Based Layered Compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Martínez-Ferrero, E.; van Smaalen, S. Incommensurate Nature of the Multilayered Molecular Ferromagnetic Metals Based on Bis(Ethylenedithio)Tetrathiafulvalene and Bimetallic Oxalate Complexes. Inorg. Chem. 2004, 43, 4808–4810. [Google Scholar] [CrossRef]
- Uji, S.; Shinagawa, H.; Terashima, T.; Yakabe, T.; Terai, Y.; Tokumoto, M.; Kobayashi, A.; Tanaka, H.; Kobayashi, H. Magnetic-Field-Induced Superconductivity in a Two-Dimensional Organic Conductor. Nature 2001, 410, 908–910. [Google Scholar] [CrossRef]
- Coronado, E.; Day, P. Magnetic Molecular Conductors. Chem. Rev. 2004, 104, 5419–5448. [Google Scholar] [CrossRef]
- Mori, T. Structural Genealogy of BEDT-TTF-Based Organic Conductors I. Parallel Molecules: Beta and Beta Phases. Bull. Chem. Soc. Jpn. 1998, 71, 2509–2526. [Google Scholar] [CrossRef]
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, M.R.; Kurmoo, M.; Day, P. Determining the Charge Distribution in BEDT-TTF Salts. Synth. Met. 1997, 86, 1973–1974. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Khasanov, S.S.; Zorina, L.V.; Buravov, L.I.; Tkacheva, V.A.; Baskakov, A.A.; Morgunov, R.B.; Gener, M.; Canadell, E.; Shibaeva, R.P.; et al. Molecular Metals Based on BEDT-TTF Radical Cation Salts with Magnetic Metal Oxalates as Counterions: B″-(BEDT-TTF)4A[M(C2O4)3]·DMF (A = NH4+, K+; M = CrIII, FeIII). Adv. Funct. Mater. 2003, 13, 403–411. [Google Scholar] [CrossRef]
- Rashid, S.; Turner, S.S.; Day, P.; Howard, J.A.K.; Guionneau, P.; McInnes, E.J.L.; Mabbs, F.E.; Clark, R.J.H.; Firth, S.; Biggs, T. New Superconducting Charge-Transfer Salts (BEDT-TTF)4[A·M(C2O4)3]·C6H5NO2 (A = H3O Or NH4, M = Cr Or Fe, BEDT-TTF = Bis(Ethylenedithio)Tetrathiafulvalene). J. Mater. Chem. 2001, 11, 2095–2101. [Google Scholar] [CrossRef]
- Turner, S.S.; Day, P.; Malik, K.M.A.; Hursthouse, M.B.; Teat, S.J.; MacLean, E.J.; Martin, L.; French, S.A. Effect of Included Solvent Molecules on the Physical Properties of the Paramagnetic Charge Transfer Salts β″-(BEDT-TTF)4[(H3O)Fe(C2O4)3]·solvent (BEDT-TTF = Bis(Ethylenedithio)Tetrathiafulvalene). Inorg. Chem. 1999, 38, 3543–3549. [Google Scholar] [CrossRef]
- Akutsu-Sato, A.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Day, P. Suppression of Superconductivity in a Molecular Charge Transfer Salt by Changing Guest Molecule: β″-(BEDT-TTF)4[(H3O)Fe(C2O4)3](C6H5CN)x(C5H5N)1-x. J. Mater. Chem. 2007, 17, 2497–2499. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Korobenko, A.V.; Zverev, V.N. Effect of Electrocrystallization Medium on Quality, Structural Features, and Conducting Properties of Single Crystals of the (BEDT-TTF)4AI[FeIII(C2O4)3]·G. CrystEngComm 2011, 13, 537–545. [Google Scholar] [CrossRef]
- Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Bulanchuk, P.O.; Zverev, V.N.; Canadell, E.; Prokhorova, T.G.; Yagubskii, E.B. Structural Phase Transition in the β″-(BEDT-TTF)4H3O[Fe(C2O4)3]·G Crystals (Where G is a Guest Solvent Molecule). CrystEngComm 2012, 14, 460–465. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Shibaeva, R.P.; Canadell, E. Effect of Halopyridine Guest Molecules on the Structure and Superconducting Properties of β″-[Bis(Ethylenedithio)tetra thiafulvalene]4(H3O)[Fe(C2O4)3]·Guest Crystals. Eur. J. Inorg. Chem. 2015, 2015, 5611–5620. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J. The Series of Molecular Conductors and Superconductors ET4[AFe(C2O4)3]·PhX (ET = Bis(Ethylenedithio)Tetrathiafulvalene; (C2O4)2− = Oxalate; A+ = H3O+, K+; X = F, Cl, Br, and I): Influence of the Halobenzene Guest Molecules on the Crystal Structure and Superconducting Properties. Inorg. Chem. 2012, 51, 1111–1126. [Google Scholar]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J. A Novel Paramagnetic Molecular Superconductor Formed by Bis(Ethylenedithio)Tetrathiafulvalene, Tris(Oxalato) Ferrate(III) Anions and Bromobenzene as Guest Molecule: ET4[(H3O)Fe(C2O4)3]·C6H5Br. J. Mater. Chem. 2005, 15, 1429–1436. [Google Scholar] [CrossRef]
- Sun, S.Q.; Wu, P.J.; Zhang, Q.C.; Zhu, D.B. The New Semiconducting Magnetic Charge Transfer Salt (BEDT-TTF)4·H2O·Fe(C2O4)3·C6H5NO2: Crystal Structure and Physical Properties. Mol. Cryst. Liq. Cryst. 1998, 319, 259–269. [Google Scholar] [CrossRef]
- Zorina, L.; Prokhorova, T.; Simonov, S.; Khasanov, S.; Shibaeva, R.; Manakov, A.; Zverev, V.; Buravov, L.; Yagubskii, E. Structure and Magnetotransport Properties of the New Quasi-Two-Dimensional Molecular Metal β″-(BEDT-TTF)4H3O[Fe(C2O4)3]·C6H4Cl2. J. Exp. Theor. Phys. 2008, 106, 347–354. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Mabbs, F.E.; McInnes, E.J.L. New Molecular Superconductor Containing Paramagnetic Chromium(III) Ions. Chem. Commun. 1997, 1367–1368. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Malik, K.M.A.; Coles, S.J.; Hursthouse, M.B. Polymorphism Based on Molecular Stereoisomerism in Tris(Oxalato) Cr(III) Salts of BEDT-TTF [Bis(Ethylenedithio)Tetrathiafulvalene]. Chem. Commun. 1999, 513–514. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J. New Magnetic Conductors and Superconductors Based on BEDT-TTF and BEDS-TTF. Synth. Met. 2005, 154, 245–248. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Shibaeva, R.P.; Buravov, L.I. Specific Structural Disorder in an Anion Layer and its Influence on Conducting Properties of New Crystals of the (BEDT-TTF)4A+[M3+(Ox)3]G Family, Where G is 2-Halopyridine; M is Cr, Ga; A+ is [K0.8(H3O)0.2]+. Crystals 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Turner, S.S.; Le Pevelen, D.; Day, P.; Light, M.E.; Hursthouse, M.B.; Firth, S.; Clark, R.J.H. β″-(BEDT-TTF)4[(H3O)Cr(C2O4)3]CH2Cl2: Effect of Included Solvent on the Structure and Properties of a Conducting Molecular Charge-Transfer Salt. Inorg. Chem. 2001, 40, 5304–5306. [Google Scholar] [CrossRef]
- Akutsu, H.; Akutsu-Sato, A.; Turner, S.S.; Le Pevelen, D.; Day, P.; Laukhin, V.; Klehe, A.; Singleton, J.; Tocher, D.A.; Probert, M.R.; et al. Effect of Included Guest Molecules on the Normal State Conductivity and Superconductivity of β′-(ET)4[(H3O)Ga(C2O4)3]·G (G = Pyridine, Nitrobenzene). J. Am. Chem. Soc. 2002, 124, 12430–12431. [Google Scholar] [CrossRef] [Green Version]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.V.; Shibaeva, R.P.; Zverev, V.N. Metallic Bi- and Monolayered Radical Cation Salts Based on Bis(Ethylenedithio) tetrathiafulvalene (BEDT-TTF) with the Tris(Oxalato)Gallate Anion. Eur. J. Inorg. Chem. 2014, 3933–3940. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Canadell, E.; Shibaeva, R.P.; Yagubskii, E.B. The First Molecular Superconductor Based on BEDT-TTF Radical Cation Salt with Paramagnetic Tris(Oxalato)Ruthenate Anion. Crystengcomm 2013, 15, 7048–7055. [Google Scholar] [CrossRef]
- Martin, L.; Morritt, A.L.; Lopez, J.R.; Nakazawa, Y.; Akutsu, H.; Imajo, S.; Ihara, Y.; Zhang, B.; Zhang, Y.; Guo, Y. Molecular Conductors from Bis(Ethylenedithio)Tetrathiafulvalene with Tris(Oxalato)Rhodate. Dalton Trans. 2017, 46, 9542–9548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benmansour, S.; Sánchez-Máñez, Y.; Gómez-García, C.J. Mn-Containing Paramagnetic Conductors with Bis(Ethylenedithio)Tetrathiafulvalene (BEDT-TTF). Magnetochemistry 2017, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Turner, S.S.; Day, P.; Light, M.E.; Hursthouse, M.B.; Firth, S.; Clark, R.J.H. The First Molecular Charge Transfer Salt Containing Proton Channels. Chem. Commun. 2001, 1462–1463. [Google Scholar] [CrossRef]
- Akutsu-Sato, A.; Akutsu, H.; Turner, S.S.; Day, P.; Probert, M.R.; Howard, J.A.K.; Akutagawa, T.; Takeda, S.; Nakamura, T.; Mori, T. The First Proton-Conducting Metallic Ion-Radical Salts. Angew. Chem. Int. Ed. 2005, 44, 292–295. [Google Scholar] [CrossRef]
- Morritt, A.L.; Lopez, J.R.; Blundell, T.J.; Canadell, E.; Akutsu, H.; Nakazawa, Y.; Imajo, S.; Martin, L. 2D Molecular Superconductor to Insulator Transition in the β″-(BEDT-TTF)2[(H2O)(NH4)2M(C2O4)3] ·18-Crown-6 Series (M = Rh, Cr, Ru, Ir). Inorg. Chem. 2019, 58, 10656–10664. [Google Scholar] [CrossRef]
- Martin, L.; Lopez, J.R.; Akutsu, H.; Nakazawa, Y.; Imajo, S. Bulk Kosterlitz–Thouless Type Molecular Superconductor β″-(BEDT-TTF)2[(H2O)(NH4)2Cr(C2O4)3]·18-Crown-6. Inorg. Chem. 2017, 56, 14045–14052. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Morritt, A.L.; Lopez, J.R.; Akutsu, H.; Nakazawa, Y.; Imajo, S.; Ihara, Y. Ambient-Pressure Molecular Superconductor with a Superlattice Containing Layers of Tris(Oxalato)Rhodate Enantiomers and 18-Crown-6. Inorg. Chem. 2017, 56, 717–720. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Day, P.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Bingham, A.; Hursthouse, M.B.; McMillan, P.; Firth, S. Multi-Layered Molecular Charge-Transfer Salts Containing Alkali Metal Ions. J. Mater. Chem. 2007, 17, 3324–3329. [Google Scholar] [CrossRef]
- Akutsu, H.; Akutsu-Sato, A.; Turner, S.S.; Day, P.; Canadell, E.; Firth, S.; Clark, R.J.H.; Yamada, J.; Nakatsuji, S. Superstructures of Donor Packing Arrangements in a Series of Molecular Charge Transfer Salts. Chem. Commun. 2004, 10, 18–19. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Hursthouse, M.B.; McMillan, P.; et al. Metallic Molecular Crystals Containing Chiral or Racemic Guest Molecules. CrystEngComm 2007, 9, 865–867. [Google Scholar] [CrossRef]
- Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Zverev, V.N.; Canadell, E.; Prokhorova, T.G.; Yagubskii, E.B. Coexistence of Two Donor Packing Motifs in the Stable Molecular Metal α-Pseudo-κ-(BEDT-TTF)4(H3O)[Fe(C2O4)3]·C6H4Br2. CrystEngComm 2011, 13, 2430–2438. [Google Scholar] [CrossRef]
- Martin, L.; Akutsu, H.; Horton, P.N.; Hursthouse, M.B.; Harrington, R.W.; Clegg, W. Chiral Radical-Cation Salts of BEDT-TTF Containing a Single Enantiomer of Tris(Oxalato)Aluminate(III) and -chromate(III). Eur. J. Inorg. Chem. 2015, 1865–1870. [Google Scholar] [CrossRef]
- Martin, L.; Akutsu, H.; Horton, P.N.; Hursthouse, M.B. Chirality in Charge-Transfer Salts of BEDT-TTF of Tris(Oxalato)Chromate(III). CrystEngComm 2015, 17, 2783–2790. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Day, P.; Horton, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H. Chiral Conducting Salts of BEDT-TTF Containing a Single Enantiomer of Tris(Oxalato)Chromate(III) Crystallised from a Chiral Solvent. J. Mater. Chem. 2010, 20, 2738–2742. [Google Scholar] [CrossRef]
- Martin, L.; Engelkamp, H.; Akutsu, H.; Nakatsuji, S.; Yamada, J.; Horton, P.; Hursthouse, M.B. Radical-Cation Salts of BEDT-TTF with Lithium Tris(Oxalato)Metallate(Iii). Dalton Trans. 2015, 44, 6219–6223. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Day, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H.; Horton, P. A Molecular Charge Transfer Salt of BEDT-TTF Containing a Single Enantiomer of Tris(Oxalato)Chromate(III) Crystallised from a Chiral Solvent. CrystEngComm 2010, 12, 1369–1372. [Google Scholar] [CrossRef]
- Benmansour, S.; Gómez-García, C.J. A Heterobimetallic Anionic 3,6-Connected 2D Coordination Polymer Based on Nitranilate as Ligand. Polymers 2016, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Benmansour, S.; Gómez-Claramunt, P.; Vallés-García, C.; Mínguez Espallargas, G.; Gómez García, C.J. Key Role of the Cation in the Crystallization of Chiral Tris(Anilato)Metalate Magnetic Anions. Cryst. Growth Des. 2016, 16, 518–526. [Google Scholar] [CrossRef]
- Benmansour, S.; Vallés-García, C.; Gómez-Claramunt, P.; Mínguez Espallargas, G.; Gómez-García, C.J. 2D and 3D Anilato-Based Heterometallic M(I)M(III) Lattices: The Missing Link. Inorg. Chem. 2015, 54, 5410–5418. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Barnett, S.A.; Tocher, D.A.; Horton, P.N.; Hursthouse, M.B. Magnetic Molecular Charge-Transfer Salts Containing Layers of Water and Tris(Oxalato)Ferrate(III) Anions. CrystEngComm 2008, 10, 192–196. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Liu, F.; Guo, Y. Synthesis, Crystal Structure, and Characterization of Charge-Transfer Salt: (BEDT-TTF)5[Fe(C2O4)3]·(H2O)2·CH2Cl2 (BEDT-TTF = Bis(Ethylenedithio) Tetrathiafulvalene). CrystEngComm. 2009, 11, 2523–2528. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Guionneau, P.; Howard, J.A.K.; Uruichi, M.; Yakushi, K. Synthesis, Crystal Structure and Properties of the Semiconducting Molecular Charge-Transfer Salt (BEDT-TTF)2Ge(C2O4)3·PhCN [BEDT-TTF = Bis(Ethylenedithio)Tetrathiafulvalene]. J. Mater. Chem. 1999, 9, 2731–2736. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H.; Horton, P.N. BEDT-TTF Tris(Oxalato)Germanate(IV) Salts with Novel Donor Packing Motifs. Bull. Chem. Soc. Jpn. 2010, 83, 419–423. [Google Scholar] [CrossRef]
- Lopez, J.R.; Akutsu, H.; Martin, L. Radical-Cation Salt with Novel BEDT-TTF Packing Motif Containing Tris(Oxalato)Germanate(IV). Synth. Met. 2015, 209, 188–191. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Bandow, S.; Maruyama, Y.; Wang, X.; Zhu, D. Physical and Structural Properties of a New Organic Conductor (BEDT-TTF)4Cu(C2O4)2. Synth. Met. 1991, 44, 147–157. [Google Scholar] [CrossRef]
- Qian, M.; Rudert, R.; Luger, P.; Ge, C.; Wang, X. Structure of the 1:4 Complex of Bis[1,2-Oxalato(2-)] Copper(II) and Bis(Ethylenedithio)Tetrathiafulvalene (BEDT-TTF). Acta Cryst. C 1991, 47, 2358–2362. [Google Scholar] [CrossRef]
- Rashid, S.; Turner, S.S.; Day, P.; Light, M.E.; Hursthouse, M.B. Molecular Charge-Transfer Salt of BEDT-TTF [Bis(Ethylenedithio)Tetrathiafulvalene] with the Oxalate-Bridged Dimeric Anion [Fe2(C2O4)5]4−. Inorg. Chem. 2000, 39, 2426–2428. [Google Scholar] [CrossRef]
- Clemente-Leon, M.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Fabre, J.M. Molecular Conductors Based upon TTF-Type Donors and Octahedral Magnetic Complexes. Synth. Met. 1999, 103, 2279–2282. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J. Charge Transfer Salts of Tetrathiafulvalene Derivatives with Magnetic Iron(III) Oxalate Complexes: [TTF]7[Fe(Ox)3]2·4H2O, [TTF]5[Fe2(Ox)5]·2PhMe·2H2O and [TMTTF]4[Fe2(Ox)5]·PhCN ·4H2O (TMTTF = Tetramethyltetrathiafulvalene). J. Chem. Soc. Dalton Trans. 2000, 205–210. [Google Scholar] [CrossRef]
- Alberola, A.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Martínez-Ferrero, E.; Murcia-Martínez, A. Multifunctionality in Hybrid Molecular Materials: Design of Ferromagnetic Molecular Metals. Synth. Met. 2003, 135, 687–689. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Zhu, D. (BEDT-TTF)3Cu2(C2O4)3(CH3OH)2: An Organic-Inorganic Hybrid Antiferromagnetic Semiconductor. Chem. Commun. 2012, 48, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Alberola, A.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Romero, F.M. Multifunctionality in Hybrid Molecular Materials: Design of Ferromagnetic Molecular Metals and Hybrid Magnets. Synth. Met. 2003, 133, 509–513. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Martínez-Agudo, J.M.; Martinez-Ferrero, E. Magnetic Properties of Hybrid Molecular Materials Based on Oxalato Complexes. Polyhedron 2003, 22, 2381–2386. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Ruiz-Pérez, C.; Triki, S. Hybrid Molecular Materials Formed by Alternating Layers of Bimetallic Oxalate Complexes and Tetrathiafulvalene Molecules: Synthesis, Structure, and Magnetic Properties of TTF4{Mn(H2O)2[Cr(Ox)3]2}.14H2O. Adv. Mater. 1996, 8, 737–740. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J.; Ruiz-Perez, C. Hybrid organic/inorganic Molecular Materials Formed by Tetrathiafulvalene Radicals and Magnetic Trimeric Clusters of Dimetallic Oxalate-Bridged Complexes: The Series (TTF)4{MII(H2O)2[MIII(Ox)3]2}·nH2O (MII = Mn, Fe, Co, Ni, Cu and Zn; MIII = Cr and Fe; Ox = C2O42−). Eur. J. Inorg. Chem. 2003, 2290–2298. [Google Scholar] [CrossRef]
- Mori, T.; Mori, H.; Tanaka, S. Structural Genealogy of BEDT-TTF-Based Organic Conductors—II. Inclined Molecules: Theta, Alpha, and Chi Phases. Bull. Chem. Soc. Jpn. 1999, 72, 179–197. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J.; Alberola, A. Radical Salts of Bis(Ethylenediseleno)Tetrathiafulvalene with Paramagnetic Tris(Oxalato)Metalate Anions. Inorg. Chem. 2006, 45, 10815–10824. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Gao, S.; Guo, Y.; Liu, F.; Zhu, D. BETS3[Cu2(C2O4)3](CH3OH)2: An Organic-Inorganic Hybrid Antiferromagnetic Metal (BETS = Bisethylene(Tetraselenfulvalene)). CrystEngComm 2013, 15, 3529–3535. [Google Scholar] [CrossRef]
- Alberola, A.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J. A Molecular Metal Ferromagnet from the Organic Donor Bis(Ethylenedithio)Tetraselenafulvalene and Bimetallic Oxalate Complexes. J. Am. Chem. Soc. 2003, 125, 10774–10775. [Google Scholar] [CrossRef]
- Awheda, I.; Krivickas, S.J.; Yang, S.; Martin, L.; Guziak, M.A.; Brooks, A.C.; Pelletier, F.; Le Kerneau, M.; Day, P.; Horton, P.N.; et al. Synthesis of New Chiral Organosulfur Donors with Hydrogen Bonding Functionality and their First Charge Transfer Salts. Tetrahedron 2013, 69, 8738–8750. [Google Scholar] [CrossRef] [Green Version]









































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benmansour, S.; Gómez-García, C.J. The Peter Day Series of Magnetic (Super)Conductors. Magnetochemistry 2021, 7, 93. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry7070093
Benmansour S, Gómez-García CJ. The Peter Day Series of Magnetic (Super)Conductors. Magnetochemistry. 2021; 7(7):93. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry7070093
Chicago/Turabian StyleBenmansour, Samia, and Carlos J. Gómez-García. 2021. "The Peter Day Series of Magnetic (Super)Conductors" Magnetochemistry 7, no. 7: 93. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry7070093