Fate of Nitrogen from Artichoke (Cynara cardunculus L. var. scolymus (L.)) Crop Residues: A Review and Lysimeter Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lysimeter Site History and Experimental Conditions
2.2. Experimental Design
2.3. Production, Analysis and Incorporation of 15N-Labeled Artichoke Plants
2.4. Crop Rotations and Practices on Lysimeters
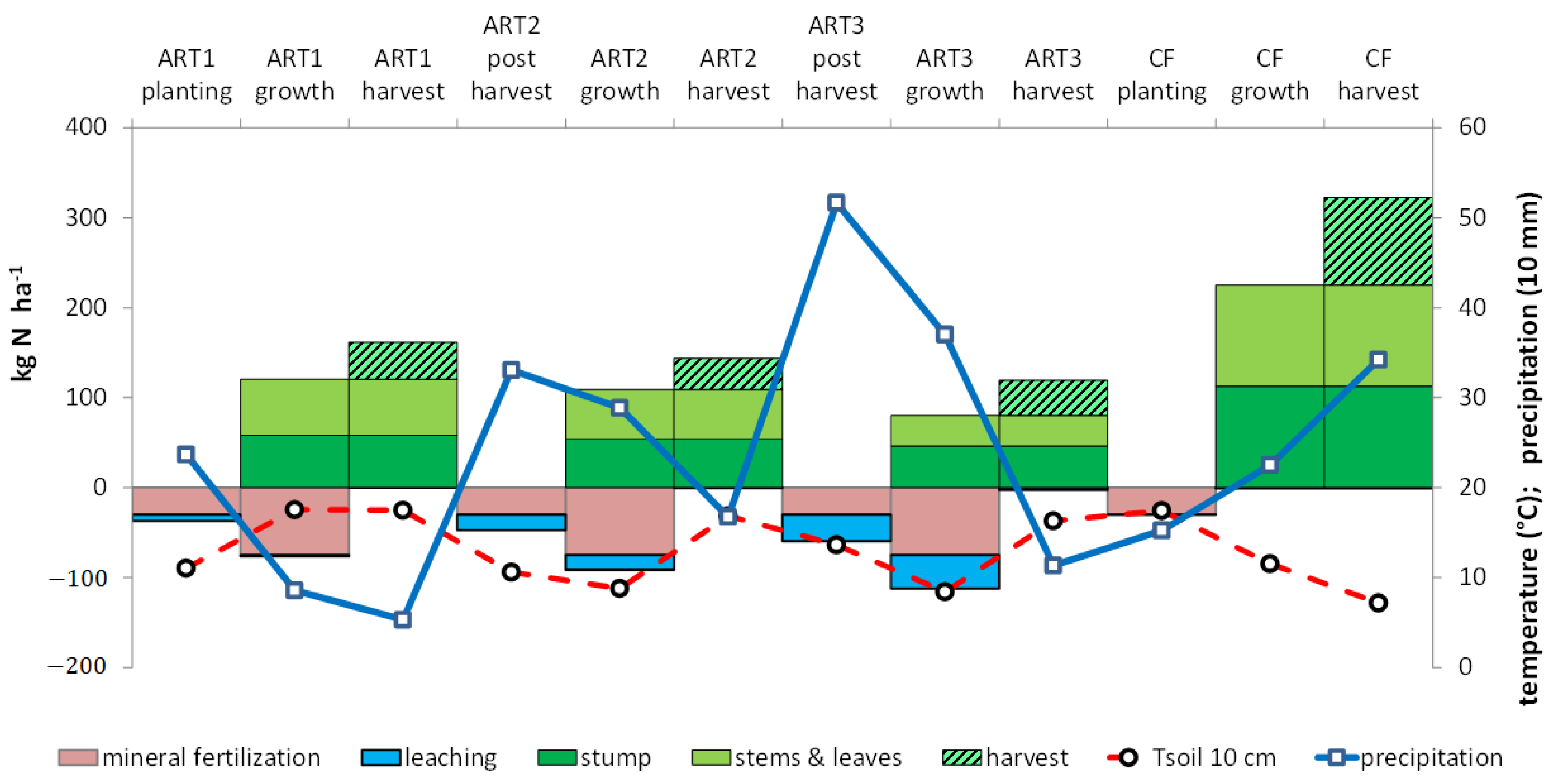
2.5. Weather
2.6. Water Sampling and Analytical Procedures
2.7. Calculations of 15N Recovery and Leaching in Plants and Water
- NX is the amount of N measured in compartment X (kg N ha−1 for a given period; e.g., harvest, water drainage);
- (atom% excess)X is the percentage of 15N in compartment X above the natural abundance in the atmosphere (reference material considered to have 0.3663 atom% of 15N atoms out of the total number of N atoms in the sample).
3. Results
3.1. Crop Production and Biomass
3.2. Nitrogen Uptake
3.3. Uptake of 15N
3.4. Water Drainage and Nitrate Leaching
3.5. Recovery of N at the Rotation Scale
4. Discussion
4.1. Yields and N Accumulation by Crops
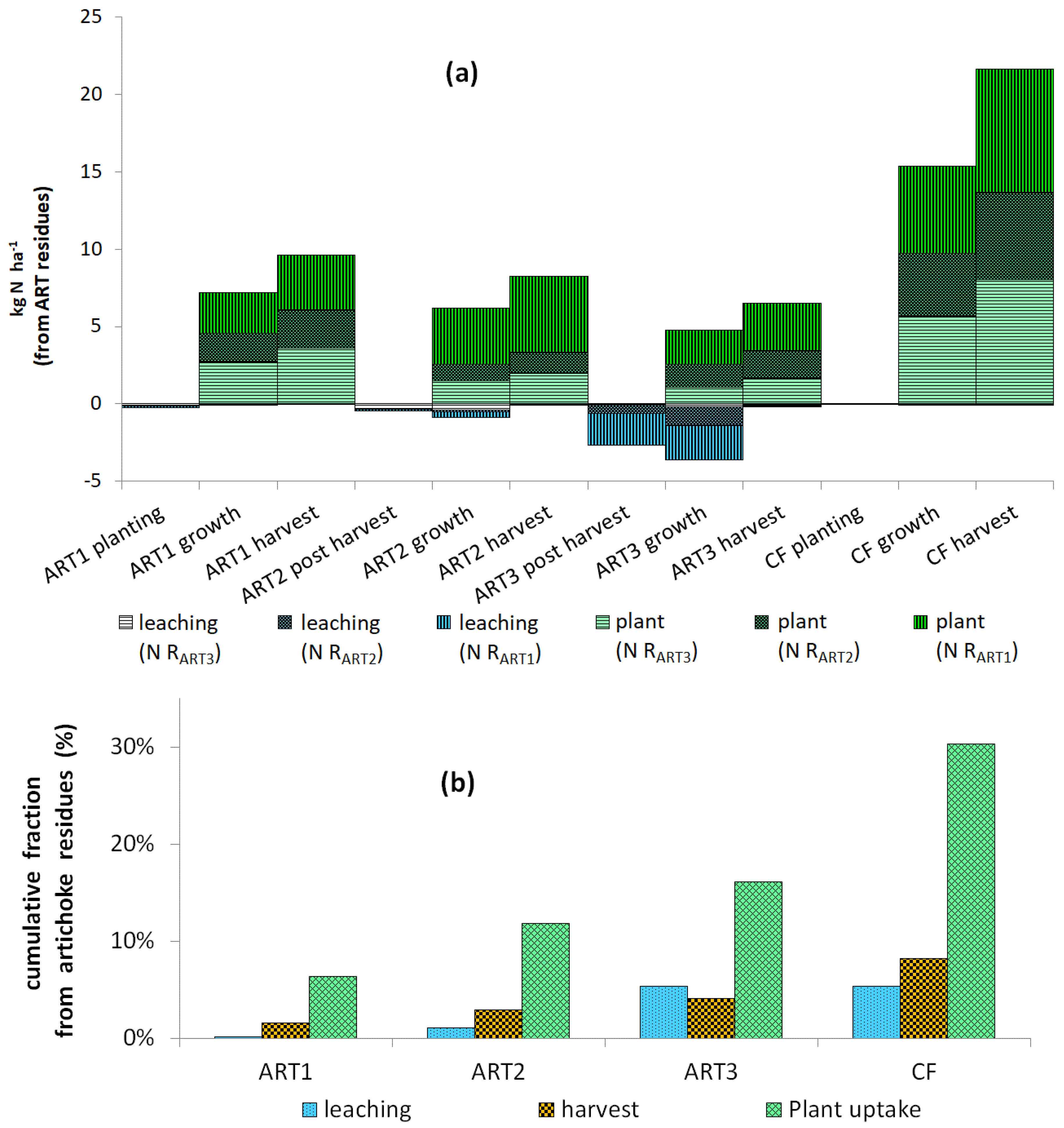
4.2. Recovery of 15N from Residues by Crops
4.3. Residual 15N Remaining in the Lysimeter after Harvest
4.4. Leaching of 15N
4.5. Reducing N Leaching by Improving Synchronization between Residue Mineralization and Crop Uptake
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Schlesinger, W.H.; Levy, H.; Michaels, A.; Schnoor, J.L. Nitrogen fixation: Anthropogenic enhancement-environmental response. Glob. Biogeochem Cycles 1995, 9, 235–252. [Google Scholar] [CrossRef] [Green Version]
- Sebilo, M.; Mayer, B.; Nicolardot, B.; Pinay, G.; Mariotti, A. Long-term fate of nitrate fertilizer in agricultural soils. Proc. Natl. Acad. Sci. USA 2013, 110, 18185–18189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crews, T.E.; Poples, M.B. Can the synchrony of nitrogen supply and crop demand be improved in legume and fertilizer-based agroecosystems? A review. Nutr. Cycl. Agroecosyst. 2005, 72, 101–120. [Google Scholar] [CrossRef]
- Akkal-Corfini, N.; Morvan, T.; Menasseri-Aubry, S.; Bissuel-Bélaygue, C.; Poulain, D.; Orsini, F.; Leterme, P. Nitrogen mineralization, plant uptake and nitrate leaching following the incorporation of (15N)-labeled cauliflower crop residues (Brassica oleracea) into the soil: A 3-year lysimeter study. Plant Soil. 2010, 328, 17–26. [Google Scholar] [CrossRef]
- Lanteri, S.; Acquadro, A.; Comino, C.; Mauro, R.; Mauromicale, G.; Portis, E. A first linkage map of globe artichoke (Cynara cardunculus var. scolymus L.) based on AFLP, S-SAP, M-AFLP and microsatellite markers. Theor. Appl. Genet. 2006, 112, 1532–1542. [Google Scholar] [CrossRef]
- Azza, A.; El-din, E.; Eman, E.; Aziz, S.F.; Hendawy, E.; Omer, A. Impact of phosphorus nutrition and number of cuttings on growth, yield and active constituents of artichoke. Int. J. Acad. Res. 2010, 2, 240–244. [Google Scholar]
- Iapichino, G. Micropropagation of globe artichoke (Cynara cardunculus L. var. scolymus). In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Lambardi, M., Ozudogru, E.A., Jain, S.M., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 994, pp. 369–380. [Google Scholar]
- FAOSTAT. Statistical Database; FAO: Rome, Italy, 2019; Available online: http://faostat.fao.org/en/?#data/QC (accessed on 4 November 2020).
- Giorgi, D.; Pandozy, G.; Farina, A.; Grosso, V.; Lucretti, S.; Crinò, P.; Saccardo, F. Karyotype of globe artichoke (Cynara cardunculus var. scolymus): Preliminary studies to define its chromosome morphology. Acta Horticulturae 2013, 133–138. [Google Scholar] [CrossRef]
- Agreste, Statistique Agricole Annuelle. Available online: http://agreste.agriculture.gouv.fr (accessed on 26 November 2020).
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Svecova, E.; Rea, E.; Lucini, L. Effects of saline stress on mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon genotypes grown in floating system. J. Sci. Food Agric. 2012, 93, 1119–1127. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knodler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Ruta, C.; Tagarelli, A.; Campanelli, A.; De Mastro, G.; Morone-Fortunato, I. Callogenesis Capability of Artichoke (Cynara cardunculus var. scolymus L. Fiori). Acta Horticulturae 2013, 377–380. [Google Scholar] [CrossRef]
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Soliman, G.; Saad, T.M. Effect of cynara scolymus L. (artichoke) extract on lipid profile of hyperlipidemic male rats. Egypt. J. Hosp. Med. 2009, 37, 733–741. [Google Scholar]
- Gominho, J.; Lourenco, A.; Palma, P.; Lourenco, M.E.; Curt, M.D.; Fernandez, J.; Pereira, H. Large scale cultivation of Cynara cardunculus L. for biomass production—A case study. Ind. Crop. Prod. 2011, 33, 1–6. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Phenolic composition and antioxidant capacity of cultivated artichoke, Madeira cardoon and artichoke-based dietary supplements. Food Res. Int. 2012, 48, 712–724. [Google Scholar] [CrossRef]
- Archontoulis, S.V.; Struik, P.C.; Vos, J.; Danalatos, N.G. Phenological growth stages of Cynara cardunculus: Codification and description according to the BBCH scale. Ann. Appl. Biol. 2010, 156, 253–270. [Google Scholar] [CrossRef]
- Duarte, D.; Figueiredo, R.; Pereira, S.; Pissarra, J. Structural characterization of the stigma style complex of Cynara cardunculus (Asteraceae) and immunolocalization of cardosins A and B during floral development. Can. J. Bot. 2006, 84, 737–749. [Google Scholar] [CrossRef]
- Angelini, L.G.; Ceccarini, L.; Nassi o Di Nasso, N.; Bonari, E. Long-term evaluation of biomass production and quality of two cardoon (Cynara cardunculus L.) cultivars for energy use. Biomass Bioenergy 2009, 33, 810–816. [Google Scholar] [CrossRef]
- Rincon, L.; Perez, A.; Pellicer, C.; Abadia, A.; Saez, J. Nutrient uptake by artichoke. Acta Horticulturae 2007, 287–292. [Google Scholar] [CrossRef]
- Archontoulis, S.V.; Vos, J.; Yin, X.; Bastiaans, L.; Danalatos, N.G.; Struik, P.C. Temporal dynamics of light and nitrogen vertical distributions in canopies of sunflower, kenaf and cynara. Field Crop Res. 2011, 122, 186–198. [Google Scholar] [CrossRef]
- Mahmoud, E.K.; Abd EL-Kader, N.K. How the nitrogen fertilization dose affects the biochemical composition and net mineralization of the artichoke residues. J. Soil Sci. Plant Nutr. 2012, 12, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Thompson, T.L.; Doerge, T.A.; Godin, R.E. Nitrogen and water interactions in subsurface drip-irrigated cauliflower: I. Plant response. Soil Sci. Soc. Am. J. 2000, 64, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.P.; Han, C.W.; Han, F.X. Organic C and N mineralization as affected by dissolved organic matter in paddy soils of subtropical China. Geoderma 2010, 157, 206–213. [Google Scholar] [CrossRef]
- Chaves, B.; De Neve, S.; Boeckx, P.; Berko, C.; Van Cleemput, O.; Hofman, G. Manipulation the N release from 15N labelled celery residues by using straw and vinasses. Soil Biol. Biochem. 2006, 38, 2244–2254. [Google Scholar] [CrossRef]
- De Neve, S.; Hofman, G. Modelling N mineralization of vegetable crop residues during laboratory incubation. Soil Biol. Biochem. 1996, 28, 1451–1457. [Google Scholar] [CrossRef]
- Rahn, C.R.; Paterson, C.D.; Vaidyanathan, L.V. The use of measurement of soil mineral N in understanding the response of crops to fertilizer nitrogen in intensive cropping rotations. J. Agric. Sci. 1998, 130, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Neeteson, J.J.; Carton, O.T. The environmental impact of nitrogen in field vegetable production. Acta Hort. 2001, 563, 21–28. [Google Scholar] [CrossRef]
- Rahn, C.R.; Bending, G.D.; Tuner, M.K. Management of N mineralization from crop residues of high N content using amendment materials of varying quality. Soil Use Manag. 2003, 19, 193–200. [Google Scholar] [CrossRef]
- Danalatos, N.G.; Skoufogianni, E.; Giannoulis, K.; Archontoulis, S.V. Responses of Cynara cardunculus to irrigation and N-fertilization in central Greece 2007. In Proceedings of the 15th European Biomass Conference & Exhibition from Research to Market Deployment, Berlin, Germany, 7–11 March 2007; Available online: http://www.researchgate.net/publication/272942878_Responses_of_Cynara_cardunculus_to_irrigation_and_N-fertilization_in_central_Greece (accessed on 25 January 2021).
- Dourado-Neto, D.; Powlson, D.; Abu Bakar, R.; Bacchi, O.O.S.; Basanta, M.V.; Cong, P.; Thi Keerthisinghe, G.; Ismaili, M.; Rahman, S.M.; Reichardt, K.; et al. Multiseason recoveries of organic and inorganic nitrogen-15 in tropical cropping systems. Soil Sci. Soc. Am. J. 2010, 74, 139–152. [Google Scholar] [CrossRef]
- Jensen, E.S. Availability of nitrogen in 15N-labelled mature pea residues to subsequent crops in the field. Soil Biol. Biochem. 1994, 26, 465–472. [Google Scholar] [CrossRef]
- Stevenson, F.C.; Walley, F.L.; van Kessel, C. Direct vs. indirect nitrogen-15 approaches to estimate nitrogen contributions from crop residues. Soil Sci. Soc. Am. J. 1998, 62, 1327–1334. [Google Scholar] [CrossRef]
- Hood, R.C.; Merckx, R.; Jensen, E.S.; Powlson, D.; Matijevic, M.; Hardarson, G. Estimating crop N uptake from organic residues using a new approach to the 15N isotope dilution technique. Plant Soil. 2000, 223, 33–46. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M.; Scott, W.R.; Frampton, C.M. Effects of N-15-labelled crop residues and management practices on subsequent winter wheat yields, nitrogen benefits and recovery under field conditions. J. Agric. Sci. 2001, 136, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Jackson, L.E. Fates and losses of nitrogen from a nitrogen-15-labeled cover crop in an intensively managed vegetable system. Soil Sci. Soc. Am. J. 2000, 64, 1404–1412. [Google Scholar] [CrossRef]
- Zapata, F.; Hera, C. Enhancing nutrient management through use of isotope techniques. In Nuclear Techniques in Soil-Plant Studies for Sustainable Agriculture and Environmental Preservation, Proceedings of the International Symposium on Nuclear and Related Techniques in Soil-Plant Studies on Sustainable Agriculture and Environmental Preservation. Vienna, Austria, 17–21 October 1994; IAEA: Vienna, Austria, 1995. [Google Scholar]
- Lehmann, J.; Muraoka, T. Tracer methods to assess nutrient uptake distribution in multistrata agroforestry systems. Agrofor. Syst. 2001, 53, 133–140. [Google Scholar] [CrossRef]
- Azam, F.; Malik, K.A.; Sajjad, M.I. Transformations in soil and availability to plants of is N applied as inorganic fertilizer and legume residues. Plant Soil. 1985, 86, 3–13. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. In World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 203. Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 21 December 2020).
- Brooks, P.D.; Stark, J.M.; McInteer, B.B.; Preston, T. Diffusion method to prepare soil extracts for automated nitrogen-15 analysis. Soil Sci. Soc. Am. J. 1989, 53, 1707–1711. [Google Scholar] [CrossRef]
- Sparling, G.P.; Zhu, C.Y.; Fillery, I.R.P. Microbial immobilization of 15N from legume residues in soils of differing textures: Measurement by persulphate oxidation and ammonia diffusion methods. Soil Biol. Biochem. 1996, 28, 1707–1715. [Google Scholar] [CrossRef]
- Morvan, T. Quantification et Modélisation des Flux D’azote Résultant des Épandages de Lisier. Ph.D. Thesis, University of Paris VI, Paris, France, 1999; 146p. [Google Scholar]
- Portela, S.I.; Andriulo, A.E.; Sasal, M.C.; Bruno, M.; Jobbagy, E.G. Fertilizer vs. organic matter contributions to nitrogen leaching in cropping systems of the Pampas: 15N application in field lysimeters. Plant Soil. 2006, 289, 265–277. [Google Scholar] [CrossRef]
- Archontoulis, S.; Danalatos, N.G.; Struik, P.; Vos, J.; Yin, X. Agronomy of Cynara cardunculus growing on an aquic soil in central Greece. In Proceedings of the International Conference on Agricultural Engineering, Hersonissos, Crete, Greece, 23–25 June 2008; pp. 1–15. Available online: https://www.researchgate.net/profile/SV_Archontoulis/publication/37790122_Agronomy_of_Cynara_cardunculus_growing_in_an_aquic_soil_in_central_Greece/links/02bfe51397069bfd8f000000.pdf (accessed on 25 January 2021).
- Shinohara, T.; Agehara, S.; Yoo, K.S.; Leskovar, D.I. Irrigation and nitrogen management of artichoke: Yield, head quality, and phenolic content. Hortscience 2011, 46, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Haggar, J.P.; Tanner, E.V.J.; Beer, J.W.; Kass, D.C.L. Nitrogen dynamics of tropical agroforestry and annual cropping systems. Soil Biol. Biochem. 1993, 25, 1363–1378. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Swift, M.J.; Merckx, R. Soil litter dynamics and N use in a leucaena (Leucaena leucocephala Lam. (DeWitt)) alley cropping system in southwestern Nigeria. Soil Biol. Biochem. 1996, 28, 739–749. [Google Scholar] [CrossRef]
- Macdonald, A.J.; Poulton, P.R.; Stockdale, E.A.; Powlson, D.S.; Jenkinson, D.S. The fate of residual 15N-labelled fertilizer in arable soils: Its availability to subsequent crops and retention in soil. Plant Soil 2002, 246, 123–137. [Google Scholar] [CrossRef]
- Vázquez, N.; Pardo, A.; Suso, M.L.; Quemada, M. A methodology for measuring drainage and nitrate leaching in unevenly irrigated vegetable crops. Plant Soil 2005, 269, 297–308. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Nwoke, O.C.; Sanginga, N.; Merckx, R. Impact of residual quality on the C and N mineralization of leaf and root residues of three agroforestry species. Plant Soil 1996, 183, 221–231. [Google Scholar] [CrossRef]
- Hart, P.B.S.; Powlson, D.S.; Poulton, P.R.; Johnson, A.E.; Jenkinson, D.S. The availability of the nitrogen in the crop residues of winter wheat to subsequent crops. J. Agric. Sci. 1993, 121, 355–362. [Google Scholar] [CrossRef]
- Glendining, M.J.; Poulton, P.R.; Powlson, D.S.; Macdonald, A.J.; Jenkinson, D.S. Availability of the residual nitrogen from a single application of 15N-labelled fertilizer to subsequent crops in a long-term continuous barley experiment. Plant Soil 2001, 233, 231–239. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Crop residues and management: Effects on soil quality, soil nitrogen dynamics, crop yield and nitrogen recovery. Adv. Agron. 2000, 68, 198–320. [Google Scholar]
- Seneviratne, G. Litter quality and nitrogen release in tropical agriculture: A synthesis. Biol. Fertil. Soils 2000, 31, 60–64. [Google Scholar] [CrossRef]
- Van Kessel, J.S.; Reeves, J.B.; Meisinger, J.J. Nitrogen and carbon mineralization of potential manure components. J. Environ. Qual. 2000, 29, 1669–1677. [Google Scholar] [CrossRef]
- Qian, P.; Schoenau, J. Availability of nitrogen in solid manure amendments with different C:N ratios. Can. J. Soil Sci. 2002, 82, 219–225. [Google Scholar] [CrossRef]
- Jensen, E.S. Dynamics of mature pea residue nitrogen turnover in unplanted soil under field conditions. Soil Biol. Biochem. 1994, 26, 455–464. [Google Scholar] [CrossRef]
- McKenney, D.J.; Wang, S.W.; Drury, C.F.; Findlay, W.I. Denitrification, immobilization and mineralization in nitrate limited and non-limited residue-amended soil. Soil Sci. Soc. Am. 1995, 59, 118–124. [Google Scholar] [CrossRef]
- Macdonald, A.J.; Poulton, P.; Powlson, D.S.; Jenkinson, D.S. Effects of season, soil type and cropping on recoveries, residues and losses of 15N-labelled fertilizer applied to arable crops in the spring. J. Agric. Sci. 1997, 129, 125–154. [Google Scholar] [CrossRef]
- Sanchez, P.A.; Jama, B. Soil fertility replenishment takes off in East and southern Africa. In Integrated Plant Nutrient Management in Sub-Saharan Africa: From Concept to Practice; Vanlauwe, B., Ed.; CAB Int.: Wallingford, UK, 2002; pp. 23–46. [Google Scholar]
- Stevens, W.B.; Hoeft, R.G.; Mulvaney, R.L. Fate of nitrogen-15 in a long-term nitrogen rate study: II. Nitrogen uptake efficiency. Agron. J. 2005, 97, 1046–1053. [Google Scholar] [CrossRef]
- Janzen, H.H.; Bole, J.B.; Biederbeck, V.O.; Slinkard, E. Fate of N applied as green manure or ammonium sulfate fertilizer to soil subsequently cropped with spring wheat in three sites in western Canada. Can. J. Soil Sci. 1990, 70, 313–323. [Google Scholar] [CrossRef]
- Ladd, J.N.; Amato, M. The fate of nitrogen from legume and fertilizer sources in soils successively cropped with wheat under field conditions. Soil Biol. Biochem. 1986, 18, 417–425. [Google Scholar] [CrossRef]
- Thönnissen, C.; Midmore, D.J.; Ladha, J.K.; Olk, D.C.; Schmidhalter, U. Legume decomposition and nitrogen release when applied as green manures to tropical vegetable production systems. Agron. J. 2000, 92, 253–260. [Google Scholar] [CrossRef]
- Swift, M.J.; Woomer, P. Organic matter and the sustainability of agricultural systems: Definition and measurement. In Soil Organic Matter Dynamics and Sustainability of Tropical Agriculture; Mulongoy, K., Merckx, R., Eds.; John Wiley & Sons: Chichester, UK, 1993; pp. 3–18. [Google Scholar]
- Min, J.; Zhang, H.L.; Shi, W.M. Optimizing nitrogen input to reduce nitrate leaching loss in greenhouse vegetable production. Agr. Water Manag. 2012, 111, 53–59. [Google Scholar] [CrossRef]
- Yang, X.L.; Lu, Y.L.; Tong, Y.A.; Yin, X.F. A 5-year lysimeter monitoring of nitrate leaching from wheat–maize rotation system: Comparison between optimum N fertilization and conventional farmer N fertilization. Agric. Ecosyst. Environ. 2015, 199, 34–42. [Google Scholar] [CrossRef]
- Giller, K.E.; Cadish, G. Future benefits from biological nitrogen fixation: An ecological approach to agriculture. Plant Soil 1995, 174, 255–277. [Google Scholar] [CrossRef]

| Lysimeter | Labeled Residues | Artichoke Part | N (% DM) | C/N Ratio | 15N (δ% Excess) | DM (g m−2) | N (kg N ha−1) |
|---|---|---|---|---|---|---|---|
| L1 | RART1 | leaves | 2.29 | 19.9 | 1.95 | 315 | 72 |
| stems | 0.71 | 71.0 | 1.72 | 341 | 24 | ||
| L2 | RART1 | leaves | 2.13 | 20.5 | 2.34 | 292 | 62 |
| stems | 0.67 | 57.7 | 2.11 | 377 | 25 | ||
| L3 | RART2 | leaves | 1.77 | 23.3 | 1.19 | 266 | 47 |
| stems | 0.48 | 80.6 | 0.98 | 197 | 9 | ||
| L4 | RART2 | leaves | 1.89 | 20.0 | 1.95 | 197 | 37 |
| stems | 0.59 | 70.2 | 1.73 | 150 | 9 | ||
| L5 | RART3 | leaves | 1.75 | - | 2.02 | 272 | 48 |
| stems | 0.44 | - | 1.82 | 209 | 9 | ||
| L6 | RART3 | leaves | 1.74 | 23.9 | 2.62 | 310 | 54 |
| stems | 0.55 | 77.6 | 2.54 | 269 | 15 |
| Lysimeter | Planting Date | Harvest Date | Residues Provided | Crop Rotation | Total FM (t ha−1) | Yield FM (t ha−1) | Aboveground Total DM (t ha−1) | Stump DM (t ha−1) | Crop Residue DM (t ha−1) | Aboveground Residues in Total DM (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| L1 | September 1998 | July 1999 | RART1 | ART2 | 26.3 | 9.3 | 4.9 | - | 3.1 | 62.5 |
| July 1999 | June 2000 | ART3 | 13.6 | 4.2 | 2.2 | 3.9 * | 1.6 | 72.8 | ||
| July 2000 | February 2001 | CF | 74.8 | 24.6 | 5.9 | - | 3.8 | 64.5 | ||
| March 2001 | September 2001 | ART1 | 44.9 | 11.2 | 9.1 | 6.8 | 7.2 | 78.6 | ||
| L2 | October 1999 | July 2000 | RART1 | ART2 | 24.4 | 8.8 | 4.4 | - | 2.8 | 63.8 |
| July 2000 | June 2001 | ART3 | 16.2 | 9.2 | 4.4 | 5.3 * | 1.9 | 43.5 | ||
| July 2001 | February 2002 | CF | 67.2 | 25.1 | 7.2 | - | 4.6 | 63.9 | ||
| March 2002 | September 2002 | ART1 | 38.7 | 12.4 | 8.8 | 6.1 | 5.9 | 67.3 | ||
| L3 | July 1999 | June 2000 | RART2 | ART3 | 12.1 | 5.0 | 2.5 | 4.4 * | 1.5 | 61.3 |
| July 2000 | February 2001 | CF | 76.1 | 24.4 | 6.2 | - | 4.2 | 67.1 | ||
| March 2001 | September 2001 | ART1 | 42.1 | 14.9 | 9.6 | - | 6.8 | 70.9 | ||
| September 2001 | July 2002 | ART2 | 49.6 | 12.2 | 10.0 | 9.2 | 7.8 | 78.3 | ||
| L4 | July 2000 | June 2001 | RART2 | ART3 | 15.3 | 7.4 | 3.9 | 6.5 * | 2.3 | 58.0 |
| July 2001 | February 2002 | CF | 54.1 | 21.0 | 6.0 | - | 3.9 | 63.9 | ||
| March 2002 | September 2002 | ART1 | 33.1 | 11.5 | 8.2 | - | 6.0 | 73.7 | ||
| September 2002 | July 2003 | ART2 | 30.5 | 10.0 | 7.5 | 9.6 | 5.3 | 70.7 | ||
| L5 | July 2000 | February 2001 | RART3 | CF | 73.3 | 26.7 | 5.6 | - | 3.4 | 60.7 |
| March 2001 | September 2001 | ART1 | 42.2 | 13.1 | 8.7 | - | 6.4 | 73.6 | ||
| September 2001 | July 2002 | ART2 | 52.8 | 14.8 | 10.3 | - | 7.8 | 75.2 | ||
| July 2002 | July 2003 | ART3 | 29.3 | 14.2 | 6.7 | 7.7 * | 4.0 | 59.1 | ||
| L6 | July 2001 | February 2002 | RART3 | CF | 72.3 | 26.7 | 7.7 | - | 5.0 | 64.7 |
| March 2002 | September 2002 | ART1 | 31.5 | 10.9 | 7.1 | - | 5.2 | 73.3 | ||
| September 2002 | July 2003 | ART2 | 28.3 | 9.8 | 6.1 | - | 4.1 | 68.4 | ||
| July 2003 | July 2004 | ART3 | 26.5 | 11.5 | 7.4 | 5.8 * | 3.3 | 44.7 |
| Lysimeter | Planting Date | Harvest Date | Residues Provided | Crop Rotation | Aboveground N Uptake (kg N ha−1) | Belowground N Uptake (kg N ha−1) | N in Aboveground Residues (kg N ha−1) | N in Aboveground Residues (%) | Precipitation (mm) | Water Drainage (mm) | Mean NO3 (mg L−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | September 1998 | July 1999 | RART1 | ART2 | 72 | - | 44 | 61 | 1024 | 441 | 41.5 |
| July 1999 | June 2000 | ART3 | 57 | 31.1 * | 33 | 58 | 1060 | 520 | 87.2 | ||
| July 2000 | February 2001 | CF | 260 | - | 145 | 56 | 854 | 536 | 2.5 | ||
| March 2001 | September 2001 | ART1 | 104 | 62.0 | 70 | 67 | 447 | 225 | 24.7 | ||
| L2 | October 1999 | July 2000 | RART1 | ART2 | 60 | - | 34 | 56 | 899 | 469 | 57.6 |
| July 2000 | June 2001 | ART3 | 68 | 48.8 * | 27 | 40 | 1186 | 783 | 51.0 | ||
| July 2001 | February 2002 | CF | 185 | - | 100 | 54 | 440 | 79 | 0.8 | ||
| March 2002 | September 2002 | ART1 | 121 | 55.3 | 73 | 60 | 405 | 150 | 13.3 | ||
| L3 | July 1999 | June 2000 | RART2 | ART3 | 43 | 39.1 * | 27 | 63 | 1060 | 468 | 98.8 |
| July 2000 | February 2001 | CF | 262 | - | 150 | 57 | 854 | 510 | 3.3 | ||
| March 2001 | September 2001 | ART1 | 103 | - | 55 | 54 | 447 | 226 | 24.5 | ||
| September 2001 | July 2002 | ART2 | 119 | 77.4 | 79 | 66 | 706 | 80 | 26.7 | ||
| L4 | July 2000 | June 2001 | RART2 | ART3 | 51 | 43.8 * | 28 | 54 | 1186 | 801 | 33.1 |
| July 2001 | February 2002 | CF | 140 | - | 72 | 51 | 440 | 86 | 3.3 | ||
| March 2002 | September 2002 | ART1 | 94 | - | 57 | 60 | 405 | 159 | 12.4 | ||
| September 2002 | July 2003 | ART2 | 85 | 43.6 | 51 | 60 | 760 | 295 | 38.6 | ||
| L5 | July 2000 | February 2001 | RART3 | CF | 189 | - | 89 | 47 | 854 | 551 | 1.5 |
| March 2001 | September 2001 | ART1 | 98 | - | 54 | 55 | 447 | 226 | 18.8 | ||
| September 2001 | July 2002 | ART2 | 108 | - | 64 | 60 | 706 | 105 | 22.3 | ||
| July 2002 | July 2003 | ART3 | 102 | 47.1 * | 49 | 48 | 808 | 316 | 33.5 | ||
| L6 | July 2001 | February 2002 | RART3 | CF | 222 | - | 120 | 54 | 440 | 78 | 1.2 |
| March 2002 | September 2002 | ART1 | 100 | - | 66 | 66 | 405 | 146 | 22.9 | ||
| September 2002 | July 2003 | ART2 | 94 | - | 61 | 64 | 760 | 298 | 102 | ||
| July 2003 | July 2004 | ART3 | 118 | 67.4 * | 41 | 35 | 742 | 260 | 62.2 |
| Lysimeter | Planting Date | Harvest Date | Residues Provided | Crop Rotation | Aboveground 15N Uptake (%) | Stump 15N Uptake (%) | Aboveground 15N in Residues Restored to Soil (%) | 15N Leached (%) | 15N Export (%) | Balance Deficit (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| L1 | September 1998 | July 1999 | RART1 | ART2 | 3.20 | - | 1.91 | 0.53 | 1.29 | 98.2 |
| July 1999 | June 2000 | ART3 | 1.50 | 0.94 | 1.00 | 3.66 | 0.50 | 94.0 | ||
| July 2000 | February 2001 | CF | 5.56 | - | 3.13 | 0.046 | 2.43 | 91.6 | ||
| March 2001 | September 2001 | ART1 | 2.13 | 1.31 | 1.41 | 0.18 | 0.72 | 90.7 | ||
| L2 | October 1999 | July 2000 | RART1 | ART2 | 3.63 | - | 2.06 | 0.68 | 1.57 | 97.7 |
| July 2000 | June 2001 | ART3 | 2.43 | 1.93 | 1.00 | 5.87 | 1.42 | 90.5 | ||
| July 2001 | February 2002 | CF | 5.61 | - | 3.02 | 0.002 | 2.59 | 87.8 | ||
| March 2002 | September 2002 | ART1 | 3.25 | 1.07 | 1.98 | 0.039 | 1.27 | 86.5 | ||
| L3 | July 1999 | June 2000 | RART2 | ART3 | 1.99 | 1.01 | 1.27 | 3.99 | 0.71 | 95.3 |
| July 2000 | February 2001 | CF | 9.61 | - | 5.65 | 0.10 | 3.96 | 91.2 | ||
| March 2001 | September 2001 | ART1 | 3.09 | - | 1.64 | 0.25 | 1.45 | 89.5 | ||
| September 2001 | July 2002 | ART2 | 1.88 | 1.37 | 1.22 | 0.073 | 0.66 | 88.8 | ||
| L4 | July 2000 | June 2001 | RART2 | ART3 | 2.32 | 1.77 | 1.72 | 3.24 | 0.61 | 96.2 |
| July 2001 | February 2002 | CF | 3.86 | - | 1.89 | 0.004 | 1.96 | 94.2 | ||
| March 2002 | September 2002 | ART1 | 2.07 | - | 1.21 | 0.055 | 0.86 | 93.3 | ||
| September 2002 | July 2003 | ART2 | 1.29 | 0.60 | 0.73 | 0.54 | 0.56 | 92.2 | ||
| L5 | July 2000 | February 2001 | RART3 | CF | 6.85 | - | 3.15 | 0.006 | 3.70 | 96.3 |
| March 2001 | September 2001 | ART1 | 3.12 | - | 1.69 | 0.14 | 1.43 | 94.7 | ||
| September 2001 | July 2002 | ART2 | 1.72 | - | 1.01 | 0.070 | 0.71 | 94.0 | ||
| July 2002 | July 2003 | ART3 | 1.30 | 0.81 | 0.66 | 0.35 | 0.65 | 93.0 | ||
| L6 | July 2001 | February 2002 | RART3 | CF | 9.51 | - | 5.61 | 0.003 | 3.90 | 96.1 |
| March 2002 | September 2002 | ART1 | 4.60 | - | 3.10 | 0.122 | 1.51 | 94.5 | ||
| September 2002 | July 2003 | ART2 | 2.12 | - | 1.37 | 1.93 | 0.75 | 91.8 | ||
| July 2003 | July 2004 | ART3 | 1.70 | 1.33 | 0.61 | 0.49 | 1.08 | 90.2 |
| Lysimeter | Residues Provided | Process | Rotation | Total 15N per Rotation (2) | 15N Conserved (3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1-L2 | RART1 | ART2 | ART3 | CF | ART1 | ||||||||
| 15N uptake (1) | 4.6% | ±0.72% | 3.4% | ±1.35% | 5.6% | ±0.04% | 3.9% | ±0.62% | 17.5% | ±2.73% | 88.6% | ||
| 15N crop export | 1.4% | ±0.20% | 1.0% | ±0.65% | 2.5% | ±0.11% | 1.0% | ±0.39% | 5.9% | ±1.35% | |||
| 15N leached | 0.6% | ±0.11% | 4.8% | ±1.56% | 0.0% | ±0.03% | 0.1% | ±0.10% | 5.5% | ±1.54% | |||
| Total 15N loss | 2.0% | ±0.31% | 5.7% | ±2.21% | 2.5% | ±0.10% | 1.1% | ±0.29% | 11.4% | ±2.91% | |||
| L3-L4 | RART2 | ART3 | CF | ART1 | ART2 | ||||||||
| 15N uptake (1) | 3.5% | ±0.77% | 6.7% | ±4.07% | 3.8% | ±1.13% | 2.6% | ±0.96% | 16.6% | ±5.39% | 90.5% | ||
| 15N crop export | 0.7% | ±0.07% | 3.0% | ±1.42% | 1.2% | ±0.42% | 0.6% | ±0.07% | 5.4% | ±1.98% | |||
| 15N leached | 3.6% | ±0.53% | 0.1% | ±0.07% | 0.2% | ±0.14% | 0.3% | ±0.33% | 4.1% | ±0.41% | |||
| Total 15N loss | 4.3% | ±0.60% | 3.0% | ±1.48% | 1.3% | ±0.56% | 0.9% | ±0.26% | 9.5% | ±2.37% | |||
| L5-L6 | RART3 | CF | ART1 | ART2 | ART3 | ||||||||
| 15N uptake (1) | 8.2% | ±1.88% | 5.1% | ±1.46% | 3.1% | ±0.70% | 2.6% | ±0.65% | 19.0% | ±4.27% | 91.6% | ||
| 15N crop export | 3.8% | ±0.14% | 1.5% | ±0.05% | 0.7% | ±0.03% | 0.9% | ±0.31% | 6.9% | ±0.53% | |||
| 15N leached | 0.0% | ±0.00% | 0.1% | ±0.01% | 1.0% | ±1.31% | 0.4% | ±0.10% | 1.6% | ±1.40% | |||
| Total 15N loss | 3.8% | ±0.15% | 1.6% | ±0.04% | 1.7% | ±1.35% | 1.3% | ±0.41% | 8.4% | ±1.93% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkal-Corfini, N.; Robin, P.; Menasseri-Aubry, S.; Corson, M.S.; Sévère, J.P.; Collet, J.M.; Morvan, T. Fate of Nitrogen from Artichoke (Cynara cardunculus L. var. scolymus (L.)) Crop Residues: A Review and Lysimeter Study. Nitrogen 2021, 2, 41-61. https://0-doi-org.brum.beds.ac.uk/10.3390/nitrogen2010004
Akkal-Corfini N, Robin P, Menasseri-Aubry S, Corson MS, Sévère JP, Collet JM, Morvan T. Fate of Nitrogen from Artichoke (Cynara cardunculus L. var. scolymus (L.)) Crop Residues: A Review and Lysimeter Study. Nitrogen. 2021; 2(1):41-61. https://0-doi-org.brum.beds.ac.uk/10.3390/nitrogen2010004
Chicago/Turabian StyleAkkal-Corfini, Nouraya, Paul Robin, Safya Menasseri-Aubry, Michael S. Corson, Jean Paul Sévère, Jean Michel Collet, and Thierry Morvan. 2021. "Fate of Nitrogen from Artichoke (Cynara cardunculus L. var. scolymus (L.)) Crop Residues: A Review and Lysimeter Study" Nitrogen 2, no. 1: 41-61. https://0-doi-org.brum.beds.ac.uk/10.3390/nitrogen2010004






