The Mercapturomic Profile of Health and Non-Communicable Diseases
Abstract
:1. Brief Overview of the Mercapturate Pathway
2. The Mercapturomic Profile
3. Biological Actions of Mercapturate Pathway-Related Metabolites
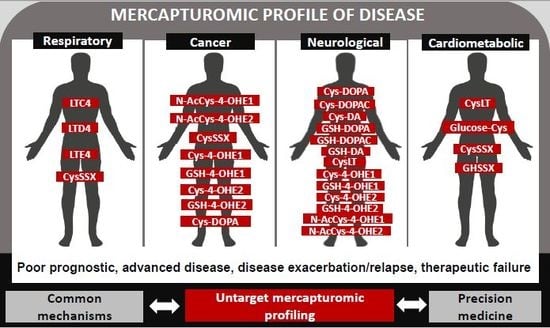
4. The Human Mercapturomic Profile in Health and Disease
4.1. Respiratory Diseases
4.2. Cancer
4.3. Neurologic Diseases
4.4. Cardiometabolic Diseases
5. Methods in Mercapturates Profiling
6. Trends and Limitations
7. Innovative Potential
Author Contributions
Funding
Conflicts of Interest
References
- Chambers, J.C.; Zhang, W.; Lord, G.M.; van der Harst, P.; Lawlor, D.A.; Sehmi, J.S.; Gale, D.P.; Wass, M.N.; Ahmadi, K.R.; Bakker, S.J.L.; et al. Genetic loci influencing kidney function and chronic kidney disease. Nat. Genet. 2010, 42, 373–375. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Veiga-da-Cunha, M.; Tyteca, D.; Stroobant, V.; Courtoy, P.J.; Opperdoes, F.R.; Van Schaftingen, E. Molecular identification of NAT8 as the enzyme that acetylates cysteine S-conjugates to mercapturic acids. J. Biol. Chem. 2010, 285, 18888–18898. [Google Scholar] [CrossRef] [PubMed]
- Szwergold, B.S. α-Thiolamines such as cysteine and cysteamine act as effective transglycating agents due to formation of irreversible thiazolidine derivatives. Med. Hypotheses 2006, 66, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Magnay, J.L.; Tong, J.; Drangova, R.; Baines, A.D. Production of cysteinyl-dopamine during intravenous dopamine therapy. Kidney Int. 2001, 59, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Ntimbane, T.; Krishnamoorthy, P.; Huot, C.; Legault, L.; Jacob, S.V.; Brunet, S.; Levy, E.; Guéraud, F.; Lands, L.C.; Comte, B. Oxidative stress and cystic fibrosis-related diabetes: A pilot study in children. J. Cyst. Fibros. 2008, 7, 373–384. [Google Scholar] [CrossRef]
- Feroe, A.G.; Attanasio, R.; Scinicariello, F. Acrolein metabolites, diabetes and insulin resistance. Environ. Res. 2016, 148, 1–6. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Hughey, R.P.; Rankin, B.B.; Elce, J.S.; Curthoys, N.P. Specificity of a particulate rat renal peptidase and its localization along with other enzymes of mercapturic acid synthesis. Arch. Biochem. Biophys. 1978, 186, 211–217. [Google Scholar] [CrossRef]
- Griffith, O.W. The role of glutathione turnover in the apparent renal secretion of cystine. J. Biol. Chem. 1981, 256, 2263–2268. [Google Scholar]
- Hanigan, M.H. γ-Glutamyl transpeptidase, a glutathionase: Its expression and function in carcinogenesis. Chem. Biol. Interact. 1998, 111, 333–342. [Google Scholar] [CrossRef]
- Commandeur, J.N.; Stijntjes, G.J.; Vermeulen, N.P. Enzymes and transport systems involved in the formation and disposition of glutathione S-conjugates. Role in bioactivation and detoxication mechanisms of xenobiotics. Pharmacol. Rev. 1995, 47, 271–330. [Google Scholar]
- Hinchman, C.A.; Rebbeor, J.F.; Ballatori, N. Efficient hepatic uptake and concentrative biliary excretion of a mercapturic acid. Am. J. Physiol. Liver Physiol. 1998, 275, G612–G619. [Google Scholar] [CrossRef] [PubMed]
- Garnier, N.; Redstone, G.G.J.; Dahabieh, M.S.; Nichol, J.N.; del Rincon, S.V.; Gu, Y.; Bohle, D.S.; Sun, Y.; Conklin, D.S.; Mann, K.K. The novel arsenical darinaparsin is transported by cystine importing systems. Mol. Pharmacol. 2014, 85, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, Y.; Boyce, J.A. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol. Res. 2014, 6, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Deng, M.; Zhang, L.; Lapus, M.G.; Hanigan, M.H. Metabolism of cisplatin to a nephrotoxin in proximal tubule cells. J. Am. Soc. Nephrol. 2003, 14, 1–10. [Google Scholar] [CrossRef]
- Stern, S.T.; Bruno, M.K.; Horton, R.A.; Hill, D.W.; Roberts, J.C.; Cohen, S.D. Contribution of acetaminophen-cysteine to acetaminophen nephrotoxicity II. Possible involvement of the γ-glutamyl cycle. Toxicol. Appl. Pharmacol. 2005, 202, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.A.; Austen, K.F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J. Clin. Investig. 1984, 73, 889–897. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pace-Asciak, C.R. Potent vasoconstriction of the isolated perfused rat kidney by leukotrienes C4 and D4. Can. J. Physiol. Pharmacol. 1983, 61, 325–328. [Google Scholar] [CrossRef]
- Badr, K.F.; Brenner, B.M.; Ichikawa, I. Effects of leukotriene D4 on glomerular dynamics in the rat. Am. J. Physiol. 1987, 253, F239–F243. [Google Scholar] [CrossRef] [PubMed]
- Shastri, S.; McNeill, J.R.; Wilson, T.W.; Poduri, R.; Kaul, C.; Gopalakrishnan, V. Cysteinyl leukotrienes mediate enhanced vasoconstriction to angiotensin II but not endothelin-1 in SHR. Am. J. Physiol. Hear. Circ. Physiol. 2001, 281, H342–H349. [Google Scholar] [CrossRef]
- Leng, W.; Kuo, C.G.; Qureshi, R.; Jakschik, B.A. Role of leukotrienes in vascular changes in the rat mesentery and skin in anaphylaxis. J. Immunol. 1988, 140, 2361–2368. [Google Scholar]
- Guo, R.; Jiang, J.; Jing, Z.; Chen, Y.; Shi, Z.; Deng, B. Cysteinyl leukotriene receptor 1 regulates glucose-stimulated insulin secretion (GSIS). Cell. Signal. 2018, 46, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; Bruno, M.K.; Hennig, G.E.; Horton, R.A.; Roberts, J.C.; Cohen, S.D. Contribution of acetaminophen-cysteine to acetaminophen nephrotoxicity in CD-1 mice: I. Enhancement of acetaminophen nephrotoxicity by acetaminophen-cysteine. Toxicol. Appl. Pharmacol. 2005, 202, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Dvash, E.; Har-Tal, M.; Barak, S.; Meir, O.; Rubinstein, M. Leukotriene C4 is the major trigger of stress-induced oxidative DNA damage. Nat. Commun. 2015, 6, 10112. [Google Scholar] [CrossRef] [PubMed]
- Salauze, L.; van der Velden, C.; Lagroye, I.; Veyret, B.; Geffard, M. Circulating antibodies to cysteinyl catecholamines in amyotrophic lateral sclerosis and Parkinson’s disease patients. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2005, 6, 226–233. [Google Scholar] [CrossRef]
- Carlsson, H.; Rappaport, S.M.; Törnqvist, M. Protein adductomics: Methodologies for untargeted screening of adducts to serum albumin and hemoglobin in human blood samples. High-Throughput 2019, 8, 6. [Google Scholar] [CrossRef]
- Wang, W.; Ballatori, N. Endogenous glutathione conjugates: Occurrence and biological functions. Pharmacol. Rev. 1998, 50, 335–356. [Google Scholar] [PubMed]
- Christ-Hazelhof, E.; Nugteren, D.H.; Van Dorp, D.A. Conversions of prostaglandin endoperoxides by glutathione-S-transferases and serum albumins. Biochim. Biophys. Acta Lipids Lipid Metab. 1976, 450, 450–461. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Haeggström, J.Z.; Funk, C.D. Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem. Rev. 2011, 111, 5866–5896. [Google Scholar] [CrossRef]
- Di Gennaro, A.; Haeggström, J.Z. The leukotrienes: Immune-modulating lipid mediators of disease. Adv. Immunol. 2012, 116, 51–92. [Google Scholar]
- Capra, V.; Thompson, M.D.; Sala, A.; Cole, D.E.; Folco, G.; Rovati, G.E. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: Critical update and emerging trends. Med. Res. Rev. 2007, 27, 469–527. [Google Scholar] [CrossRef]
- Gonçalves-Dias, C.; Morello, J.; Correia, M.; Coelho, N.; Antunes, A.M.M.; Macedo, M.P.; Monteiro, E.C.; Soto, K.; Pereira, S.A. Mercapturate pathway in the tubulocentric perspective of diabetic kidney disease. Nephron 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.; Dvash, E. Leukotrienes and kidney diseases. Curr. Opin. Nephrol. Hypertens. 2018, 27, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Gelosa, P.; Colazzo, F.; Tremoli, E.; Sironi, L.; Castiglioni, L. Cysteinyl leukotrienes as potential pharmacological targets for cerebral diseases. Mediat. Inflamm. 2017, 2017, 3454212. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.; Butler, C.T.; Murphy, A.; Moran, B.; Gallagher, W.M.; O’Sullivan, J.; Kennedy, B.N. Evaluation of cysteinyl leukotriene signaling as a therapeutic target for colorectal cancer. Front. Cell Dev. Biol. 2016, 4, 103. [Google Scholar] [CrossRef]
- Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 10 March 2019).
- Pelclová, D.; Fenclova, Z.; Vlcková, Š.; Lebedová, J.; Syslova, K.; Pecha, O.; Belacek, J.; Navrátil, T.; Kuzma, M.; Kacer, P. Leukotrienes B4, C4, D4 and E4 in the exhaled breath condensate (EBC), blood and urine in patients with pneumoconiosis. Ind. Health 2012, 50, 299–306. [Google Scholar]
- Celik, D.; Doruk, S.; Koseoglu, H.I.; Sahin, S.; Celikel, S.; Erkorkmaz, U. Cysteinyl leukotrienes in exhaled breath condensate of smoking asthmatics. Clin. Chem. Lab. Med. 2013, 51, 1069–1073. [Google Scholar] [CrossRef]
- Wennergren, G. Inflammatory mediators in blood and urine. Paediatr. Respir. Rev. 2000, 1, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Gaki, E.; Papatheodorou, G.; Ischaki, E.; Grammenou, V.; Papa, I.; Loukides, S. Leukotriene E4 in urine in patients with asthma and COPD-The effect of smoking habit. Respir. Med. 2007, 101, 826–832. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Boyce, J.A. Cysteinyl leukotriene receptors, old and new; implications for asthma. Clin. Exp. Allergy 2012, 42, 1313–1320. [Google Scholar] [CrossRef]
- Montuschi, P. LC/MS/MS analysis of leukotriene B4 and other eicosanoids in exhaled breath condensate for assessing lung inflammation. J. Chromatogr. B 2009, 877, 1272–1280. [Google Scholar] [CrossRef]
- Montuschi, P. Leukotrienes, antileukotrienes and asthma. Mini Rev. Med. Chem. 2008, 8, 647–656. [Google Scholar] [CrossRef]
- Green, S.A.; Malice, M.P.; Tanaka, W.; Tozzi, C.A.; Reiss, T.F. Increase in urinary leukotriene LTE4levels in acute asthma: Correlation with airflow limitation. Thorax 2004, 59, 100–104. [Google Scholar] [CrossRef]
- Ono, E.; Taniguchi, M.; Higashi, N.; Mita, H.; Yamaguchi, H.; Tatsuno, S.; Fukutomi, Y.; Tanimoto, H.; Sekiya, K.; Oshikata, C. Increase in salivary cysteinyl-leukotriene concentration in patients with aspirin-intolerant asthma. Allergol. Int. 2011, 60, 37–43. [Google Scholar] [CrossRef]
- Stephenson, S.T.; Brown, L.A.S.; Helms, M.N.; Qu, H.; Brown, S.D.; Brown, M.R.; Fitzpatrick, A.M. Cysteine oxidation impairs systemic glucocorticoid responsiveness in children with difficult-to-treat asthma. J. Allergy Clin. Immunol. 2015, 136, 454–461. [Google Scholar] [CrossRef]
- Shimbori, C.; Shiota, N.; Okunishi, H. Involvement of leukotrienes in the pathogenesis of silica-induced pulmonary fibrosis in mice. Exp. Lung Res. 2010, 36, 292–301. [Google Scholar] [CrossRef]
- Wimmer, I.; Meyer, J.C.; Seifert, B.; Dummer, R.; Flace, A.; Burg, G. Prognostic value of serum 5-S-cysteinyldopa for monitoring human metastatic melanoma during immunochemotherapy. Cancer Res. 1997, 57, 5073–5076. [Google Scholar]
- Banfalvi, T.; Gilde, K.; Boldizsar, M.; Fejös, Z.; Horvath, B.; Liszkay, G.; Beczassy, E.; Kremmer, T. Serum concentration of 5-S-cysteinyldopa in patients with melanoma. Eur. J. Clin. Investig. 2000, 30, 900–904. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Kageshita, T.; Furue, M.; Hatta, N.; Kiyohara, Y.; Nakayama, J.; Ono, T.; Saida, T.; Takata, M.; Tsuchida, T. Evaluation of 5-S-cysteinyldopa as a marker of melanoma progression: 10 years’ experience. Melanoma Res. 2002, 12, 245–253. [Google Scholar] [CrossRef]
- Sato, S.; Aoki, T.; Umezu, K.; Mori, M.; Hayashi, M.; Saito, H.; Kitamura, K.; Tsuchida, A.; Koyanagi, Y.; Yamagishi, T. Rectal malignant melanoma diagnosed by N-isopropyl-p-123I-iodoamphetamine single photon emission computed tomography and 5-S-cysteinyl dopa: Report of a case. Surg. Today 2003, 33, 454–458. [Google Scholar] [CrossRef]
- Umemura, H.; Yamasaki, O.; Kaji, T.; Otsuka, M.; Asagoe, K.; Takata, M.; Iwatsuki, K. Usefulness of serum 5-S-cysteinyl-dopa as a biomarker for predicting prognosis and detecting relapse in patients with advanced stage malignant melanoma. J. Dermatol. 2017, 44, 449–454. [Google Scholar] [CrossRef]
- Salehi, F.; Dunfield, L.; Phillips, K.P.; Krewski, D.; Vanderhyden, B.C. Risk factors for ovarian cancer: An overview with emphasis on hormonal factors. J. Toxicol. Environ. Heal. Part B Crit. Rev. 2008, 11, 301–321. [Google Scholar] [CrossRef]
- Zahid, M.; Beseler, C.L.; Hall, J.B.; LeVan, T.; Cavalieri, E.L.; Rogan, E.G. Unbalanced estrogen metabolism in ovarian cancer. Int. J. Cancer 2014, 134, 2414–2423. [Google Scholar] [CrossRef]
- Zahid, M.; Goldner, W.; Beseler, C.L.; Rogan, E.G.; Cavalieri, E.L. Unbalanced estrogen metabolism in thyroid cancer. Int. J. Cancer 2013, 133, 2642–2649. [Google Scholar] [CrossRef]
- Gaikwad, N.W.; Yang, L.; Muti, P.; Meza, J.L.; Pruthi, S.; Ingle, J.N.; Rogan, E.G.; Cavalieri, E.L. The molecular etiology of breast cancer: Evidence from biomarkers of risk. Int. J. Cancer 2008, 122, 1949–1957. [Google Scholar] [CrossRef]
- Gaikwad, N.W.; Yang, L.; Pruthi, S.; Ingle, J.N.; Sandhu, N.; Rogan, E.G.; Cavalieri, E.L. Urine biomarkers of risk in the molecular etiology of breast cancer. Breast Cancer Basic Clin. Res. 2009, 3, 1–8. [Google Scholar] [CrossRef]
- Gaikwad, N.W.; Yang, L.; Weisenburger, D.D.; Vose, J.; Beseler, C.; Rogan, E.G.; Cavalieri, E.L. Urinary biomarkers suggest that estrogen-DNA adducts may play a role in the aetiology of non-Hodgkin lymphoma. Biomarkers 2009, 14, 502–512. [Google Scholar] [CrossRef]
- Jonas, C.R.; Puckett, A.B.; Jones, D.P.; Griffith, D.P.; Szeszycki, E.E.; Bergman, G.F.; Furr, C.E.; Tyre, C.; Carlson, J.L.; Galloway, J.R. Plasma antioxidant status after high-dose chemotherapy: A randomized trial of parenteral nutrition in bone marrow transplantation patients. Am. J. Clin. Nutr. 2000, 72, 181–189. [Google Scholar] [CrossRef]
- Hopkins, M.H.; Fedirko, V.; Jones, D.P.; Terry, P.D.; Bostick, R.M. Antioxidant micronutrients and biomarkers of oxidative stress and inflammation in colorectal adenoma patients: Results from a randomized, controlled clinical trial. Cancer Epidemiol. Prev. Biomark. 2010, 19, 1055–9965. [Google Scholar] [CrossRef]
- Kish, S.J.; Shannak, K.; Hornykiewicz, O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic parkinsons disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 1988, 318, 876–880. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Jinsmaa, Y.; Sullivan, P.; Holmes, C.; Kopin, I.J.; Sharabi, Y. 3,4-Dihydroxyphenylethanol (hydroxytyrosol) mitigates the increase in spontaneous oxidation of dopamine during monoamine oxidase inhibition in PC12 cells. Neurochem. Res. 2016, 41, 2173–2178. [Google Scholar] [CrossRef]
- Kurth, M.C.; Adler, C.H. COMT inhibition. Neurology 1998, 50, S3–S14. [Google Scholar] [CrossRef]
- Fornstedt, B.; Brun, A.; Rosengren, E.; Carlsson, A. The apparent autoxidation rate of catechols in dopamine-rich regions of human brains increases with the degree of depigmentation of substantia nigra. J. Neural Transm. Dis. Dement. Sect. 1989, 1, 279–295. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Holmes, C.; Sullivan, P.; Jinsmaa, Y.; Kopin, I.J.; Sharabi, Y. Elevated cerebrospinal fluid ratios of cysteinyl-dopamine/3, 4-dihydroxyphenylacetic acid in parkinsonian synucleinopathies. Parkinsonism Relat. Disord. 2016, 31, 79–86. [Google Scholar] [CrossRef]
- Cheng, F.-C.; Kuo, J.-S.; Chia, L.-G.; Dryhurst, G. Elevated 5-S-cysteinyldopamine/homovanillic acid ratio and reduced homovanillic acid in cerebrospinal fluid: Possible markers for and potential insights into the pathoetiology of Parkinson’s disease. J. Neural Transm. 1996, 103, 433–446. [Google Scholar] [CrossRef]
- Carlsson, A.; Fornstedt, B. Catechol metabolites in the cerebrospinal fluid as possible markers in the early diagnosis of Parkinson’s disease. Neurology 1991, 41, 50–51. [Google Scholar]
- Goldstein, D.S.; Sullivan, P.; Holmes, C.; Miller, G.W.; Alter, S.; Strong, R.; Mash, D.C.; Kopin, I.J.; Sharabi, Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J. Neurochem. 2013, 126, 591–603. [Google Scholar] [CrossRef]
- Riederer, P.; Sofic, E.; Rausch, W.-D.; Schmidt, B.; Reynolds, G.P.; Jellinger, K.; Youdim, M.B.H. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J. Neurochem. 1989, 52, 515–520. [Google Scholar] [CrossRef]
- Jenner, P.; Dexter, D.T.; Sian, J.; Schapira, A.H.V.; Marsden, C.D. Oxidative stress as a cause of nigral cell death in Parkinson’s disease and incidental lewy body disease. Ann. Neurol. 1992, 32, S82–S87. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E.; Jenner, P.; Halliwell, B. Superoxide-dependent depletion of reduced glutathione by L-DOPA and dopamine. Relevance to parkinson’s disease. Neuroreport 1995, 6, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E.; Jenner, P.; Daniel, S.E.; Lees, A.J.; Marsden, D.C.; Halliwell, B. Conjugates of catecholamines with cysteine and GSH in Parkinson’s disease: Possible mechanisms of formation involving reactive oxygen species. J. Neurochem. 1998, 71, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, N.W.; Murman, D.; Beseler, C.L.; Zahid, M.; Rogan, E.G.; Cavalieri, E.L. Imbalanced estrogen metabolism in the brain: Possible relevance to the etiology of Parkinson’s disease. Biomarkers 2011, 16, 434–444. [Google Scholar] [CrossRef]
- Pardo, C.A.; Vargas, D.L.; Zimmerman, A.W. Immunity, neuroglia and neuroinflammation in autism. Int. Rev. Psychiatry 2005, 17, 485–495. [Google Scholar] [CrossRef]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef]
- Qasem, H.; Al-Ayadhi, L.; El-Ansary, A. Cysteinyl leukotriene correlated with 8-isoprostane levels as predictive biomarkers for sensory dysfunction in autism. Lipids Health Dis. 2016, 15, 130. [Google Scholar] [CrossRef]
- Janicka, M.; Kot-Wasik, A.; Kot, J.; Namieśnik, J. Isoprostanes-biomarkers of lipid peroxidation: Their utility in evaluating oxidative stress and analysis. Int. J. Mol. Sci. 2010, 11, 4631–4659. [Google Scholar] [CrossRef]
- Simmet, T.; Seregi, A.; Hertting, G. Formation of sulphidopeptide-leukotrienes in brain tissue of spontaneously convulsing gerbils. Neuropharmacology 1987, 26, 107–110. [Google Scholar] [CrossRef]
- Kiwak, K.J.; Moskowitz, M.A.; Levine, L. Leukotriene production in gerbil brain after ischemic insult, subarachnoid hemorrhage, and concussive injury. J. Neurosurg. 2009, 62, 865–869. [Google Scholar] [CrossRef]
- Moskowitz, M.; Kiwak, K.; Hekimian, K.; Levine, L. Synthesis of compounds with properties of leukotrienes C4 and D4 in gerbil brains after ischemia and reperfusion. Science 2006, 224, 886–889. [Google Scholar] [CrossRef]
- Winking, M.; Lausberg, G.; Simmet, T. Malignancy-dependent formation of cysteinyl-leukotrienes in human brain tumor tissues and its detection in urine. In Neurosurgical Standards, Cerebral Aneurysms, Malignant Gliomas; Piscol, K., Klinger, M., Brock, M., Eds.; Springer: Berlin, Germany, 1992; Volume 20, pp. 334–335. [Google Scholar]
- Bittl, J.A.; Pfeffer, M.A.; Lewis, R.A.; Mehrotra, M.M.; Corey, E.J.; Austen, K.F. Mechanism of the negative inotropic action of leukotrienes C4 and D4 on isolated rat heart. Cardiovasc. Res. 1985, 19, 426–432. [Google Scholar] [CrossRef]
- Winking, M.; Deinsberger, W.; Joedicke, A.; Boeker, D.K. Cysteinyl-leukotriene levels in intracerebral hemorrhage: An edema-promoting factor? Cerebrovasc. Dis. 1998, 8, 318–326. [Google Scholar] [CrossRef]
- Carry, M.; Korley, V.; Willerson, J.T.; Weigelt, L.; Ford-Hutchinson, A.W.; Tagari, P. Increased urinary leukotriene excretion in patients with cardiac ischemia: In vivo evidence for 5-lipoxygenase activation. Circulation 1992, 85, 230–236. [Google Scholar] [CrossRef]
- Allen, S.P.; Sampson, A.P.; Piper, P.J.; Chester, A.H.; Ohri, S.K.; Yacoub, M.H. Enhanced excretion of urinary leukotriene E4 in coronary artery disease and after coronary artery bypass surgery. Coron. Artery Dis. 1993, 4, 899–904. [Google Scholar] [CrossRef]
- de Prost, N.; El-Karak, C.; Avila, M.; Ichinose, F.; Melo, M.F.V. Changes in cysteinyl leukotrienes during and after cardiac surgery with cardiopulmonary bypass in patients with and without chronic obstructive pulmonary disease. J. Thorac. Cardiovasc. Surg. 2011, 141, 1496–1502. [Google Scholar] [CrossRef]
- Söder, P.Ö.; Söder, B.; Nowak, J.; Jogestrand, T. Early carotid atherosclerosis in subjects with periodontal diseases. Stroke 2005, 36, 1195–1200. [Google Scholar] [CrossRef]
- Grau, A.J.; Becher, H.; Ziegler, C.M.; Lichy, C.; Buggle, F.; Kaiser, C.; Lutz, R.; Bültmann, S.; Preusch, M.; Dörfer, C.E. Periodontal disease as a risk factor for ischemic stroke. Stroke 2004, 35, 496–501. [Google Scholar] [CrossRef]
- Persson, G.R.; Ohlsson, O.; Pettersson, T.; Renvert, S. Chronic periodontitis, a significant relationship with acute myocardial infarction. Eur. Heart J. 2003, 24, 2108–2115. [Google Scholar] [CrossRef]
- Bäck, M.; Airila-Månsson, S.; Jogestrand, T.; Söder, B.; Söder, P.-Ö. Increased leukotriene concentrations in gingival crevicular fluid from subjects with periodontal disease and atherosclerosis. Atherosclerosis 2007, 193, 389–394. [Google Scholar] [CrossRef]
- Stanke-Labesque, F.; Bä, M.; Lefebvre, B.; Tamisier, R.; Baguet, J.-P.; Arnol, N.; Lé, P.; Pé, J.-L.; Grenoble, F.; Stockholm, S. Increased urinary leukotriene E4 excretion in obstructive sleep apnea: Effects of obesity and hypoxia. J. Allergy Clin. Immunol. 2009, 124, 364–370. [Google Scholar] [CrossRef]
- Gautier-Veyret, E.; Bäck, M.; Arnaud, C.; Belaïdi, E.; Tamisier, R.; Lévy, P.; Arnol, N.; Perrin, M.; Pépin, J.-L.; Stanke-Labesque, F. Cysteinyl-leukotriene pathway as a new therapeutic target for the treatment of atherosclerosis related to obstructive sleep apnea syndrome. Pharmacol. Res. 2018, 134, 311–319. [Google Scholar] [CrossRef]
- Hardy, G.; Boizel, R.; Bessard, J.; Cracowski, J.L.; Bessard, G.; Halimi, S.; Stanke-Labesque, F. Urinary leukotriene E4 excretion is increased in type 1 diabetic patients: A quantification by liquid chromatography-tandem mass spectrometry. Prostaglandins Other Lipid Mediat. 2005, 78, 291–299. [Google Scholar] [CrossRef]
- Boizel, R.; Bruttmann, G.; Benhamou, P.Y.; Halimi, S.; Stanke-Labesque, F. Regulation of oxidative stress and inflammation by glycaemic control: Evidence for reversible activation of the 5-lipoxygenase pathway in type 1, but not in type 2 diabetes. Diabetologia 2010, 53, 2068–2070. [Google Scholar] [CrossRef]
- Dhawan, S.S.; Eshtehardi, P.; McDaniel, M.C.; Fike, L.V.; Jones, D.P.; Quyyumi, A.A.; Samady, H. The role of plasma aminothiols in the prediction of coronary microvascular dysfunction and plaque vulnerability. Atherosclerosis 2011, 219, 266–272. [Google Scholar] [CrossRef]
- Ashfaq, S.; Abramson, J.L.; Jones, D.P.; Rhodes, S.D.; Weintraub, W.S.; Hooper, W.C.; Vaccarino, V.; Harrison, D.G.; Quyyumi, A.A. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J. Am. Coll. Cardiol. 2006, 47, 1005–1011. [Google Scholar] [CrossRef]
- Rafnsson, A.; Bäck, M. Urinary leukotriene E4 is associated with renal function but not with endothelial function in type 2 diabetes. Dis. Mark. 2013, 35, 475–480. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S. Improved HPLC determination of 5-S-cysteinyldopa in serum. Clin. Chem. 1994, 40, 495–496. [Google Scholar]
- Jones, D.P.; Carlson, J.L.; Mody, V.C.; Cai, J.; Lynn, M.J.; Sternberg, P. Redox state of glutathione in human plasma. Free Radic. Biol. Med. 2000, 28, 625–635. [Google Scholar] [CrossRef]
- Ashfaq, S.; Abramson, J.L.; Jones, D.P.; Rhodes, S.D.; Weintraub, W.S.; Hooper, W.C.; Vaccarino, V.; Alexander, R.W.; Harrison, D.G.; Quyyumi, A.A. Endothelial function and aminothiol biomarkers of oxidative stress in healthy adults. Hypertension 2008, 52, 80–85. [Google Scholar] [CrossRef]
- Patel, R.S.; Ghasemzadeh, N.; Eapen, D.J.; Sher, S.; Arshad, S.; Ko, Y.; Veledar, E.; Samady, H.; Zafari, A.M.; Sperling, L. Novel biomarker of oxidative stress is associated with risk of death in patients with coronary artery disease. Circulation 2016, 133, 361–369. [Google Scholar] [CrossRef]
- Mathias, P.I.; B’Hymer, C. Mercapturic acids: Recent advances in their determination by liquid chromatography/mass spectrometry and their use in toxicant metabolism studies and in occupational and environmental exposure studies. Biomarkers 2016, 21, 293–315. [Google Scholar] [CrossRef]
- Nunes, J.; Charneira, C.; Morello, J.; Rodrigues, J.; Pereira, S.A.; Antunes, A.M.M. Mass Spectrometry-Based Methodologies for Targeted and Untargeted Identification of Protein Covalent Adducts (Adductomics): Current status and challenges. High-Throughput 2019, 8, 9. [Google Scholar] [CrossRef]


| Disease | Aim | Study Population | Mercapturomic Profile |
|---|---|---|---|
| Pneumo-coniosis | Evaluate the impact of pneumoconiosis and systemic diseases, drugs and diet on LTC4 and LTE4 levels measured in EBC, plasma and urine Ref [39] | A total of 82 patients with pneumoconiosis: 45 from asbestos exposure (mean age 70 yo; 53% men) 37 from silica exposure (mean age 69 yo; 97% men) and 27 CTLs Subjects with systemic disorders (atherosclerosis, cancer) were present in all groups | In CTLs, plasma LTE4 correlated with nephrolithiasis (+) and fibrates (+) In asbestosis, CysLTs correlated with lung function (−), plasma LTC4 correlated with steroids (+) In silicosis, urine LTD4 correlated with kidney failure (+), and salicylates (+); and plasma LTE4 with vitamin C and E (+) EBC: LTB4 asbestosis > silicosis and CTL; LTD4 and LTE4 asbestosis and silicosis > CTL. Urine: LTD4 asbestosis > CTL. Plasma: LTE4 asbestosis > CTL |
| Asthma | Evaluate the effect of smoking in LTD4 and LTE4 levels in asthma Ref [40] | EBC from 59 asthmatic patients: 30 smokers (mean age 34 yo; 50% men) and 29 non-smokers (mean age 34 yo; 48% men); and 29 CTLs (mean age 34 yo; 48% men; non-smokers) | LTD4 asthmatic smokers > asthmatic non-smokers and CTLs LTE4 asthmatic > CTL LTE4 correlated with FEV1/FVC ratio (−) |
| LTE4 levels in treatment of asthma exacerbation with CysLTR1 antagonists Ref [46] | 184 patients with acute asthma at ED (age 35 yo): 123 on ß agonist + montelukast; 61 on ß agonist + placebo. Sampling at ED and 2 weeks after | Urine: LTE4 during exacerbations > 2 weeks later. No differences in LTE4 during exacerbation or 2 weeks later in patients receiving montelukast or placebo. LTE4 correlated with FEV1 (−) during exacerbation and 2 weeks later | |
| CysLTs levels in saliva of AIA patients Ref [47] | 26 non-smoking asthmatic patients: 15 AIA (mean age 51 yo; 40% men) and 11 ATA (mean age 55 yo; 36% men); 10 CTLs; patients were also divided in mild (n = 6) and severe (n = 9) asthma | Saliva LTC4 and LTE4 AIA > ATA and CTL Urine LTE4 AIA > ATA and CTL and LTE4 severe AIA > mild AIA | |
| Characterize systemic Cys-S-conjugates that are disulfides and its association with asthma Ref [48] | Plasma and PBMCs samples from 99 children with asthma (median age 12 yo; 67% men) and 15 CTLs (median age 10 yo; 20% men). On treatment for asthma, 57 difficult-to-treat children underwent glucocorticoid responsiveness test | Plasma: CysSSCys and EhCysSH/CysSSCys in asthma > CTL. PBMCs: GSSG in asthma > CTL In glucocorticoid treated children, EhCysSH/CysSSCys in non-responders > responders before and after treatment |
| Disease | Aim | Study Population | Mercapturomic Profile |
|---|---|---|---|
| Melanoma | Usefulness of serum Cys-DOPA levels in MM prognosis and response to immuno-chemotherapy Ref [50] | Serum samples from 11 patients with MM before and after each immunochemotherapy cycle Mean age 47 yo; 64% men | Cys-DOPA MM patients after therapy > MM patients before therapy > healthy controls Patients with declines in Cys-DOPA > 68% of treatment cycles had longer survival time |
| Value of Cys-DOPA in different stages of MM Ref [51] | Serum samples from 252 patients followed for 1 to 4 months: patients with no evidence of MM after surgery or chemotherapy (asymptomatic patients), patients with MM classified according to symptoms or clinical I-III stages. Range age 18–86 yo; 51% men | Cys-DOPA symptomatic > asymptomatic patients Cys-DOPA stage III > stage I and II patients Cys-DOPA stage III > primary tumor, lymph node and lung metastasis symptoms ↑ Cys-DOPA correlated with tumor mass | |
| Usefulness of serum Cys-DOPA in melanoma progression and prognosis Ref [52] | Serum samples of 218 melanoma patients Mean age 55 yo; 51% men | Cys-DOPA > 10 nmol/L in stage IV patients Elevation of Cys-DOPA preceded or occurred at the same time of clinical detection of visceral metastasis in, respectively, 33% and 37% of cases ↑ Cys-DOPA associated with shorter survival time | |
| Case report for diagnosis of rectal malignant melanoma with Cys-DOPA Ref [53] | Serum samples of a woman 84 yo diagnosed with rectal MM | Before tumor resection: Cys-DOPA = 26 nM. Three months after surgery: Cys-DOPA = 12.6 nM (still > 10 nM) and CT scan confirmed multiple liver and lung metastasis. | |
| Usefulness of serum Cys-DOPA as a biomarker for prognosis and early detection of relapse of malignant melanoma Ref [54] | Serum samples of 120 patients with advanced stage malignant melanoma Mean age 64 yo; 41% men | Cys-DOPA in advanced stages (III and IV) > early stages (0–II) patients. In patients with advanced stages, Cys-DOPA > 15 nM correlated with a poor prognosis In 11/14 patients with melanoma recurrence, Cys-DOPA > 10 nM around the time of relapse | |
| Colorectal adenoma | Evaluate the effects of antioxidant micronutrients on oxidative and inflammatory biomarkers in sporadic colorectal adenoma. Ref [62] | Pilot, randomized, double-blind, placebo-controlled clinical trial. Plasma samples from 47 patients with a history of sporadic colorectal adenoma: 23 under placebo (median age 59 yo; 52% men) and 24 under antioxidant treatment with 800 mg vitamin E, 24 mg β-carotene, 1000 mg vitamin C, 200 μg L-selenomethionine, 7.2 mg riboflavin, 80 mg niacin, 60 mg zinc and 5 mg manganese (median age 61 yo; 50% men). | CysSSCys in the antioxidant (−39%) < placebo group In the antioxidant group, ↓ CysSSCys was only statistically significant in nonsmokers (−35%) vs. smokers (−12%) |
| Leukemia lymphoma | Determine the effect of high-dose chemotherapy and type of parenteral nutrition in circulating antioxidants in patients undergoing BMT Ref [61] | Double-blind, controlled, randomized clinical trial. Plasma samples from 24 BMT patients (mean age 40 yo; 58% men) with NHL (n = 10), chronic myeloid leukemia (n = 8), Hodgkin disease (n = 4), acute myeloid leukemia (n = 1) and T cell lymphoma (n = 1). Most patients received chemotherapy or chemotherapy + radiation. Patients were divided into treatment with standard parenteral nutrition with amino acids, dextrose, lipids, vitamins minerals and electrolytes (mean age 41 yo; 36% men) or modified parenteral nutrition (mean age 38 yo; 69% men) with electrolytes, vitamins, minerals and less lipids. Samples were collected before chemotherapy and BMT (baseline) and 1, 3, 7, 10 and 14 days after BMT. | ↑ EhGSH/GSSG, EhCys/CysSSCys and CysSSCys over time, regardless of treatment or parental nutrition type |
| non-Hodgkin lymphoma | Determine if the estrogen metabolites and adducts are involved in the etiology of NHL Ref [60] | Urine samples from 15 NHL patients (median age 59 yo; 100% men) and 30 CTLs (median age 60; 100% men). Estrogen metabolites included Cys, GSH and NAC conjugates of 4-OHE1(E2) and 2-OHE1(E2). | Cys, GSH and NAC conjugates of 4-OHE1(E2) in CTL > NHL group |
| Thyroid cancer | Investigate the role of estrogen metabolites and adducts in thyroid cancer Ref [57] | Urine samples from 40 women with thyroid cancer (mean age 47 yo) and 40 CTL women (mean age 47 yo). The estrogen metabolites included Cys, GSH and NAC conjugates of 4-OHE1(E2) and 2-OHE1(E2). | Ratio depurinating estrogen-DNA adducts to estrogen metabolites and S-conjugates in thyroid cancer > CTL group |
| Ovarian cancer | Investigate the role of estrogen metabolites and adducts in ovarian cancer Ref [56] | Urine samples from 33 women with ovarian cancer (mean age 58 yo) and 34 CTL women (mean age 58 yo). The ratio of depurinating estrogen DNA adducts to estrogen metabolites and S-conjugates was obtained. The estrogen metabolites included Cys, GSH and NAC conjugates of 4-OHE1(E2) and 2-OHE1(E2). | Ratio depurinating estrogen DNA adducts to estrogen metabolites and S-conjugates in ovarian cancer > CTL. |
| Breast cancer | Evaluate the urinary levels of estrogen metabolites and adducts in breast cancer | Urine samples from 12 women with high-risk for breast cancer (mean age 52 yo), 17 with breast cancer (mean age 54 yo) and 46 CTLs (mean age 50 yo). The ratio of depurinating estrogen DNA adducts to metabolites was obtained. Estrogen metabolites included Cys, GSH and NAC conjugates of 4-OHE1(E2) and 2-OHE1(E2). Ref [58] | Cys, GSH and NAC conjugates of 2-OHE1(E2) in CTL > other groups. |
| Urine samples from 40 women with high-risk for breast cancer (median age 57 yo); 40 with newly diagnosed breast cancer (median age 58 yo); CTLs (median age 45 yo). All without estrogen-containing treatments. The ratio of depurinating estrogen DNA adducts to metabolites was obtained. Estrogen metabolites included Cys, GSH and NAC conjugates of 4-OHE1(E2) and 2-OHE1(E2). Ref [59] | Cys, GSH and NAC conjugates of 2-OHE1(E2) and 4-OHE1(E2) in CTL > other groups. Ratio in breast cancer and high-risk group for breast cancer > in CTL. |
| Disease | Aim | Study Population | Mercapturomic Profile |
|---|---|---|---|
| Parkinson | Investigate the association between SN’s degenerative changes and the occurrence of Cys-DOPA, Cys-DA and Cys-DOPAC Ref [66] | Postmortem brain samples (SN, PUT and CN sections) from 17 individuals. 72–90 yo; 41% men. Samples were divided according to degree of depigmentation and neuronal loss within SN (12 pigmented, 5 depigmented) | DOPA, DA, DOPAC depigmented < pigmented in SN. No differences for Cys-S-conjugates Cys-DA/DA, Cys-DOPAC/DOPAC depigmented > pigmented in SN Cys-DOPA/DOPA depigmented > pigmented in PUT |
| Evaluate the levels of Cys-DA and HVA in CSF samples of PD patients Ref [68] | CSF samples from 20 PD patients (mean age 69 yo, 85% men) and 16 CTLs (mean age 60 yo years, 63 % men); Samples under and 5 days after L-DOPA withdrawal | HVA in PD patients after L-DOPA withdrawal < CTLs. No differences for Cys-DA among groups. Cys-DA/HVA in PD after L-DOPA withdrawal > CTLs. | |
| Assess Cys- and GSH-conjugates of DA, DOPA and DOPAC in brain tissue and changes on their levels in PD Ref [74] | Postmortem brain samples from six PD patients with PD (mean age 77 yo, L-DOPA therapy) and six CTLs (mean age 81 yo); Brains were dissected into 11 regions | Detectable conjugates in most brain regions, with higher levels in SN and PUT GSH-conjugates < Cys-conjugates Cys-conjugates in SN in PD > CTLs | |
| Assess estrogen metabolites and adducts in PD Ref [75] | Urine samples from 20 PD patients (mean age 62 yo; 75% men; all under levodopa) and 40 CTLs (mean age 63 yo; 75% men).Estrogen metabolites included Cys-, GSH- and NAC- conjugates of 4-OHE1(E2) and 2-OHE1(E2) | Cys, GSH and NAC conjugates of 4-OHE1(E2) CTL > PD group | |
| Assess the value of Cys-DA/DOPAC ratio in CSF as a specific biomarker of parkinsonism Ref [67] | CSF samples from 24 PD patients (mean age 61 yo, 58% men); 32 MSA-P (mean age 60 yo; 66% men); 18 PAF (mean age 63 yo; 67% men) and 32 CTLs (mean age 53 yo; 53% men). Patients were not on levodopa or MAO inhibitors | Cys-DA levels were similar among groups; Cys-DA/DOPAC > 2-fold in PD and MSA-P than PAF and CTL groups Cys-DA/DOPAC was correlated with putamen/occipital ratios (−) and washout fractions of 18F-fluorodopa-derived radioactivity (+) | |
| Autism | Determine the correlation of 8-isoprostane, LTs, age and autism severity scales Ref [78] | Plasma samples from 44 autistic children (mean age 7 yo) and 40 CTLs (mean age 7 yo). Autistic cases were all simple and tested negative for the fragile X gene mutations *LTs measured = LTA4 + LTC4 + LTD4 + LTE4 | CysLTs and 8-isoprostane in autistic > CTL. CysLTs correlated with 8-isoprostane (+) SSP test correlated with CysLTs and 8-isoprostane (−) |
| Disease | Aim | Study Population | Mercapturomic Profile |
|---|---|---|---|
| Diabetes | To evaluate the influence of diabetes, glycaemia control and ACE inhibitor on LTE4 excretion. Ref [95] | Urine samples from 34 T1D patients: 20 with good metabolic control (age 39 yo; 55% men), 14 poor metabolic control (age 41 yo; 50% men); 28 CTLs (age 39 yo; 43% men). All nonsmokers | LTE4 T1D > CTL. LTE4 in T1D with poor metabolic control > CTL No influence of ACE Inhibitors on urinary LTE4 |
| Evaluate the effect of insulin treatment on the urinary excretion of LTE4. Ref [96] | Urine samples from 20 T1D (mean age 37 yo; 35% men) and 19 T2D patients (mean age 58 yo; 68% men). Non-smokers. Intensive insulin treatment over 3 months | ↓ LTE4 after insulin treatment (−32%) in T1D but not in T2DM | |
| Assess if Cys is a good transglycating agent. Ref [4] | Urine samples from five diabetic patients and two normoglycemic subjects | Glucose-Cys diabetes > normoglycemic subjects | |
| To evaluate the association of urinary LTE4 with endothelial function Ref [99] | Urine samples from 30 (median age 65 yo; 80% men) T2DM subjects of at least 2 years duration and eGFR 71 (14–129) mL/min | Decreased renal function associated with ↓ urinary LTE4; LTE4 associated with serum creatinine (−) and eGFR (+); eGFR was an independent predictor of urinary LTE4 levels | |
| OSA-related atherogenesis | Identify the factors influencing LTE4 levels and the role of LTE4 in OSA-related atherosclerosis Ref [94] | Urine samples from 170 OSA patients (mean age 57 yo; 81 % men): 136 CVE free and 34 with previous CVE; 29 CTLs (mean age 52 yo; 52% men): 22 CVE free and seven with previous CVE | LTE4 associated with age, min SaO2 and history of CVE and intima-media thickness. LTE4 in OSA CVE free patients > CTL CVE free group. Increase related to minSaO2 and traditional risk factors of the 10-year CDV risk score |
| OSA-related obesity | To evaluate the influence of obesity and CPAP in urinary LTE4 levels and the role of LTE4 as biomarker of inflammation in patients with OSA. Ref [93] | Urine samples from 40 non-obese OSA patients (mean age 49 yo; 85% men) and 25 CTLs (mean age 45 yo; 72% men). A group of 72 OSA patients with any BMI starting CPAP (mean age 51 yo; 81% men) was included to study confounder factors of LTE4. All nonsmokers | LTE4 in non-obese OSA > CTLs. In the 40 non-obese OSA patients, LTE4 was correlated with % of time spent with SaO2 < 90% (+). In the 72 OSA patients, BMI and % of time spent with SaO2 < 90% were identified as independent predictors of LTE4. CPAP treatment for at least 4 weeks ↓ LTE4 by 22% only in OSA patients with normal BMI |
| Intra-Cerebral hemorrhage | Quantify LTs in urine of spontaneous ICH patients and evaluate its impact in the edema formation. Ref [85] | Urine samples from 17 spontaneous ICH patients (mean age 58 yo; 53% men): 12 treated surgically and five conservatively. Sampling before treatment and during the five following days. CysLTs measured = sum of LTC4, LTD4 and LTE4 | CysLTs correlated with hematoma volume (+) CysLTs 5 days after < before surgery CysLTs did not decrease after the conservative treatment |
| Coronary artery diseases | Assess LTE4 during and after acute coronary syndromes Ref [86] | Urine samples from 16 AMI (mean age 51 yo; 88% men); 14 UA patients (mean age 52 yo; 21% men); eight clinical CTLs (non-ischemic heart pain) (88% mean) and 10 normal CTLs (50% men) CTLs (non-evidence of coronary artery disease). Samples were collected upon admission with acute chest pain and 3 days after | LTE4 in MIA and UA at admission > CTL groups LTE4 on admission > 3 days after UA |
| Study the relation between the systemic LTE4 levels and stable coronary artery disease before and after bypass surgery Ref [87] | Urine samples from 13 chronic stable angina (mean age 59 yo; 100% men) and 12 CTLs (mean age 44 yo; 100% men). Single urine patients from CTLs and patients before surgery; postoperative 24 h urine samples over seven successive days. 6/13 patients on aspirin until a few days before surgery. All but three patients on aspirin after the operation. | LTE4 in preoperative patients > CTL LTE4 2 days after > before surgery | |
| Detect the formation of CysLTs and atherosclerosis lesions in carotid artery in subjects with and without periodontitis Ref [92] | GCF samples from 19 subjects with periodontitis (mean age 55 yo; 63% men; 13 with atherosclerotic plaques in carotid artery) and 16 CTLs (mean age 53 yo; 44% men; five with atherosclerotic plaques in carotid artery). | Subjects with atherosclerotic plaques in periodontitis > in CTL CysLT higher in periodontitis with higher dental plaque index CysLTs in subjects with > subjects without atherosclerotic plaques in all subjects independently on periodontitis | |
| Coronary artery diseases | Assess oxidative stress parameters in the bloodstream as a reliable predictor of endothelial function. Ref [98] | Plasma samples from 124 healthy nonsmokers without CDV risk factors (44 yo; 40% men). At recruitment, 41 patients were HTN, diabetes or BMI ≥ 30. Vasodilation was measured at the brachial artery | CysSSCys related with age(+), BMI(+), HTN(+) Framingham score CysSSCys in HTN, diabetes or BMI ≥ 30 > remaining individuals CysSSG related with TG(−), HDL(+), HTN(+). CysSSCys and CysSSG independent predictors of endothelium-dependent vasodilation |
| Test if ↑ oxidative stress was associated with impaired coronary microvascular function and plaque necrotic core content Ref [97] | Plasma samples from 47 patients with an abnormal non-invasive stress test, stable angina or stabilized acute coronary syndrome undergoing cardiac catheterization (mean age 58 yo; 64% men). Microvascular function and epicardial plaque measured in the coronary artery | ↑ CysSSCys/GSH associated with impaired microvascular function and greater epicardial necrotic core ↑ CysSSCys in patients with ↑ BMI and HTN | |
| Study of CysLTs changes during and after cardiac surgery with CPB in patients with and without COPD Ref [88] | Patients undergoing cardiac surgery with CPB: nine moderate-to-severe COPD (69 yo; 78% men) + 10 non-smoker no COPD patients (64 yo; 60% men). Urine and plasma at baseline, end of CPB, after CPB and 2 h after admission in ICU. CysLTs = LTC4 + LTD4 + LTE4 | ↑ urine CysLTs with time in both groups, but more evident in COPD patients; Plasma Cys LTs baseline < at admission to ICU in patients with COPD | |
| Assess LTE4 during and after acute coronary syndromes Ref [86] | Urine samples from 16 AMI (mean age 51 yo; 88% men); 14 UA patients (mean age 52 yo; 21% men); eight clinical CTLs (non-ischemic heart pain) (88% mean) and 10 normal CTLs (50% men) CTLs (non-evidence of coronary artery disease). Samples collected upon admission with acute chest pain and 3 days after. | LTE4 in MIA and UA at admission > CTL groups. LTE4 on admission > 3 days after UA. |
| Analyte | Sample | Sampling and Pre-Treatment and Analyses | |
|---|---|---|---|
| 5-S-CyS-DOPA | Serum | Sampling: blood collected into plain evacuated tubes and allowed to coagulate. Pre-treatment: commercial kit (Immundiagnostik GmbH, Bensheim, Germany). Extraction and purification on acid-washed aluminum oxide. LC-EC Ref [50] | |
| Ref [51] | |||
| Sampling: blood collected into plain evacuated tubes and allowed to coagulate. Sample: 500 µL serum. Pre-treatment: adsorption of 5-S-cysteinyl-DOPA to alumina, washing alumina with a phosphate buffer pH 4.0 and elution of 5-S-cysteinyl-dopa with HC1O4. LC-EC Method from Ref [100] Ref [52,54] | |||
| Ref [53] | |||
| 5-S-CyS-DA | CSF | Sample: 70 µL CSF; Sampling: collection at 7:30 and 8:30 am, ultrafiltration into Millipore Ultrafree-MC units having a NMWC cut-off of 10,000. LC-EC Ref [68] | |
| Sample: 1 mL; batch alumina extraction; LLOD 10 pmol/L, or 10 fmol per assayed mL of CSF; LC-EC Ref [67] | |||
| 5-S-Cys-DOPA, 5-S-Cys-DA, 5-S-Cys- DOPAC 5-GSH-DOPA, 5-GSH-DA 5-GSH-DOPAC | Tissues from 11 different brain regions. | Homogenization and digestion with proteinase K in a digestion buffer with addition of perchloric acid 0.1 M. Centrifugation and supernatant filtration (0.22-pm Micropure separators). Adsorption of catechols from 100 µL of the filtrate to acid-washed aluminum oxide. Washing aluminum oxide with distilled water and elution of catechols with mobile phase (pH 2.7). LC Ref [74] | |
| Cys-4-OHE1 Cys-4-OHE2 GSH-4-OHE1 GSH-4-OHE2 N-AcCys-4-OHE1 N-AcCys -4-OHE2 | Spot urine | About 50 mL was collected from each participant and 1 mg/mL ascorbic acid was added to prevent oxidation of the catechol moieties. Urine samples (2 mL) were adjusted to pH 7 and then loaded into phenyl SPE pre-conditioned cartridges. Elution with methanol/10 mmM ammonium formate pH 7 (90:10) with 1% acetic acid. | LC-MS Normalization to urine creatinine Ref [58,59,60,75] |
| LC-MS Normalization with depurinating estrogen DNA adducts Ref [56,57] | |||
| Glucose-cysteine | Urine | No extraction. GC-MS SIM of characteristic ions 632, 745, 604 and 726 m/z. Normalization by urinary creatinine Ref [4] | |
| LTE4 | Urine | Urine 10 mL was stabilized by the addition of NaOH and 4-OH-TEMPO before freezing. LC purification. Radioimmunoassay LLOD 8 pg/mL. Normalization with urinary creatinine sulfate Ref [86] | |
| 25 mL SPE followed by LC purification. Radioimmunoassay Normalization with urinary creatinine Ref [87] | |||
| Spot urine. LC purification. Radioimmunoassay normalization with urinary creatinine LLOD: 6.3 pg of LTE 4 per milligram of creatinine Ref [46] | |||
| Ref [95] 4 mL of urine SPE LC-MS/MS in negative mode. Acquisition in MRM m/z 438.2 → 333.0. Normalization with urinary creatinine | |||
| Ref [93] Urine collection at 7:00 am. Pre-treatment SPE LC-MS/MS in negative mode. Acquisition in MRM m/z 438.2 → 333.0 LLOD: 10 pg/mL urine. Normalization with urinary creatinine | |||
| Ref [96] Overnight urine collection. Pre-treatment SPE; C-MS/MS in negative mode. Acquisition in MRM m/z 438.2 → 333.0 LLOD: 10 pg/mL urine. Normalization with urinary creatinine | |||
| Ref [99] 50 uL urine No purification of sample required. EIA Range: 7.8–1000 pg/mL. Normalization by urinary creatinine | |||
| Ref [94] Sample collection at 7:00 am. SPE MS/MS in negative mode. Acquisition in MRM m/z 438.2 → 333.0 LLOD: 10 pg/mL urine. Normalization with urinary creatinine | |||
| Sum of LTC4, LTD4 LTE4 | Urine | Ref [85] Urine samples were added to 8 mL methanol containing 4-hydroxy-TEMPO (4- hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) and ethylenediami- netetraacedic acid (EDTA) in final concentrations of 1.0 and 0.5 mM, respectively before freezing. Pre-treatment 2 mL SPE followed by LC purification. Radioimmunoassay normalization with urinary creatinine | |
| Urine Saliva | 1 mL saliva SPE followed by LC purification. EIA Ref [47] | ||
| Sum of LTC4, LTD4 LTE4 | GSF | EIA; LLOD 7.8 pg/mL for LTC4 + LTD4 + LTE4 and 3.9 pg/mL for LTB4 Ref [92] | |
| LTD4 LTE4 | EBC | At least 1.5 mL of EBC. ELISA Ref [40] | |
| Sum of LTB4, LTC4 LTD4, LTE4 | EBC Plasma Urine | EBC (1 mL); Blood and a spot urine samples were taken between 8:00 and 12:00 a.m. Blood collected with EDTA. SPE. LC-MS/MS Ref [39] | |
| Plasma Urine | SPE for plasma samples. Enzyme-linked immunosorbent assay. Urinary LTs normalized with urinary creatinine. Plasma LTs concentrations were corrected for changes in plasma protein concentration Ref [88] | ||
| Sum of LTA4, LTC4 LTD4, LTE4 | Plasma | Blood collected overnight fasting in EDTA tubes. Plasma. SPE. ELISA Ref [78] | |
| CysSSCys CySSG | Plasma | Precipitation of potassium perchlorate with KOH/tetraborate solution followed by derivatization with dansyl chloride. Blood samples collected after overnight fasting. Blood collected into specially prepared tubes containing a preservative solution with serine, sodium heparin, BPDS, iodoacetic acid, borate and tetraborate. The supernatant was then transferred to a perchloric acid solution before freezing LC-FD Ref [101,102] | |
| CysSSCys | Plasma | Blood collected in heparin tubes and immediately placed in preservation buffer containing BPDS. The supernatant was added to ice-cold 10% perchloric acid in 10 μmol gamma-glutamylglutamate before freezing LC-FD Ref [61] | |
| Blood collected in sodium heparin tubes and transferred into specially prepared tubes with preservative containing serine, sodium heparin, BPDS, iodoacetic acid and borate. Supernatant transferred into a tube containing 10% ice-cold perchloric acid and 0.2 M boric acid solution LC-FD Ref [97] | |||
| Blood collected in EDTA tubes. After centrifugation, butylated hydroxytoluene and salicylic acid as lipid and aqueous antioxidants were added before freezing LC-FD Ref [62] | |||
| Aliquots were preserved in a 5% perchloric acid solution containing iodoacetic acid (6.7 μmol/L) and boric acid (0.1 mol/L) before freezing LC-FD Ref [48] | |||
| Blood collected into heparin tubes and transferred into a preservative solution before freezing LC-MS Ref [103] | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves-Dias, C.; Morello, J.; Semedo, V.; Correia, M.J.; Coelho, N.R.; Monteiro, E.C.; Antunes, A.M.M.; Pereira, S.A. The Mercapturomic Profile of Health and Non-Communicable Diseases. High-Throughput 2019, 8, 10. https://0-doi-org.brum.beds.ac.uk/10.3390/ht8020010
Gonçalves-Dias C, Morello J, Semedo V, Correia MJ, Coelho NR, Monteiro EC, Antunes AMM, Pereira SA. The Mercapturomic Profile of Health and Non-Communicable Diseases. High-Throughput. 2019; 8(2):10. https://0-doi-org.brum.beds.ac.uk/10.3390/ht8020010
Chicago/Turabian StyleGonçalves-Dias, Clara, Judit Morello, Valdir Semedo, M. João Correia, Nuno R. Coelho, Emilia C. Monteiro, Alexandra M. M. Antunes, and Sofia A. Pereira. 2019. "The Mercapturomic Profile of Health and Non-Communicable Diseases" High-Throughput 8, no. 2: 10. https://0-doi-org.brum.beds.ac.uk/10.3390/ht8020010