Nitride-Coated and Anodic-Oxidized Titanium Promote a Higher Fibroblast and Reduced Streptococcus gordonii Proliferation Compared to the Uncoated Titanium
Abstract
:1. Introduction
2. Results
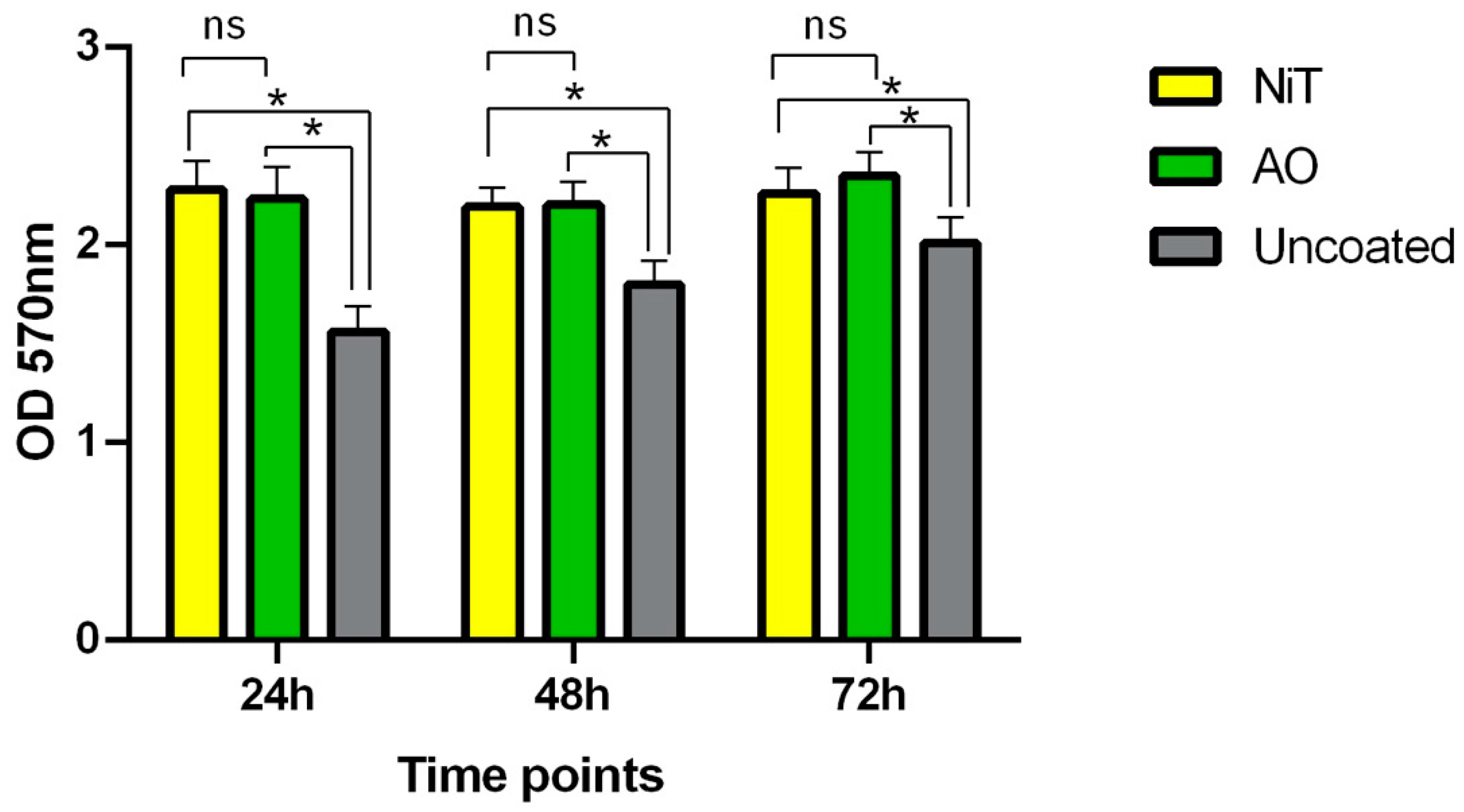
2.1. Fibroblasts Metabolic Activity and Proliferation
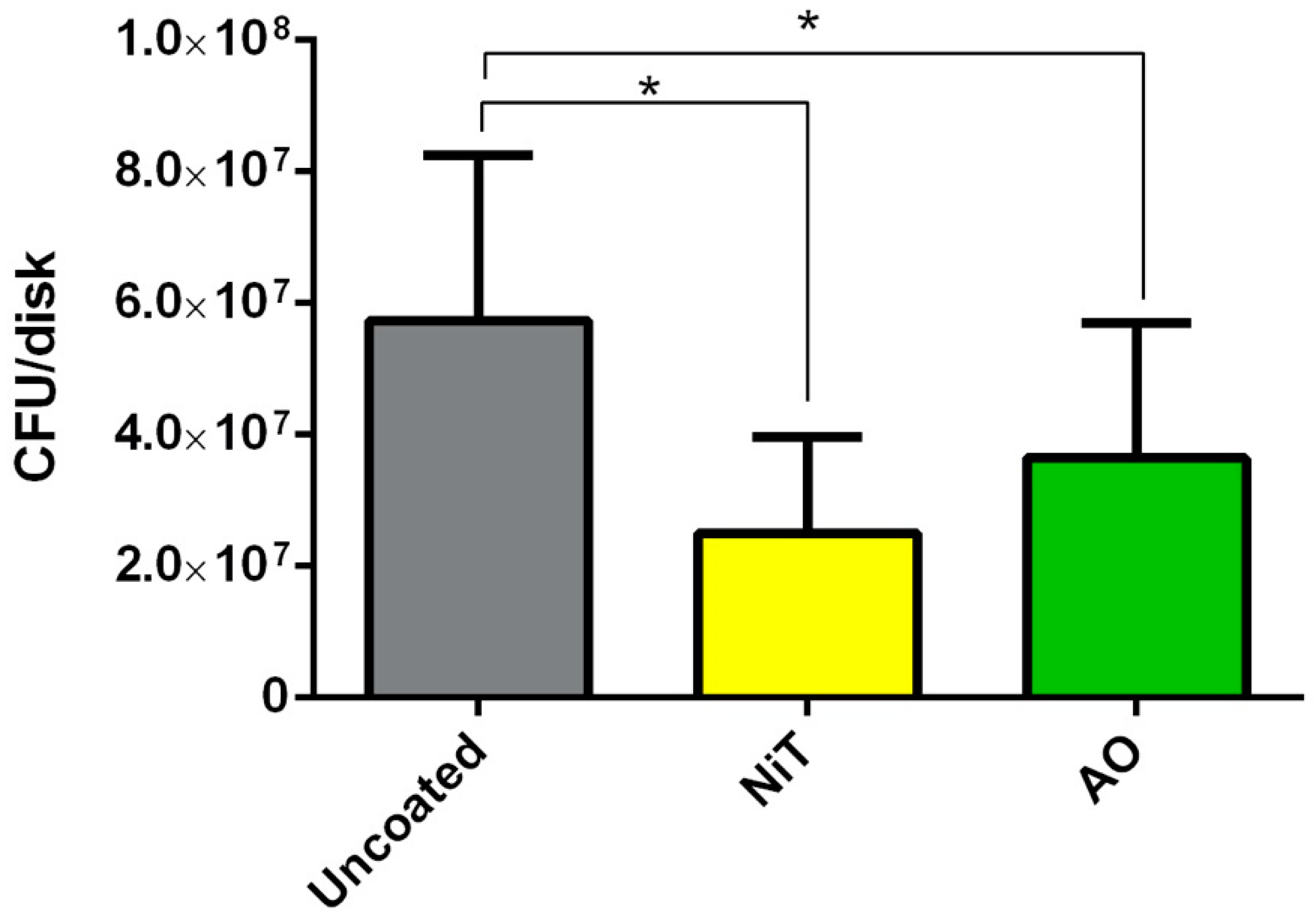
2.2. Streptococcus Gordonii Growth
3. Discussion
4. Materials and Methods
4.1. Cell Culture and MTT Assay
4.2. Biofilm Formation Experiments
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogle, O.E. Implant surface material, design, and osseointegration. Dent. Clin. N. Am. 2015, 59, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Delgado-Ruiz, R.; Sculean, A. Concepts for prevention of complications in implant therapy. Periodontol. 2000 2019, 81, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Maghaireh, H.; Grusovin, M.G.; Ziounas, I.; Worthington, H.V. Soft tissue management for dental implants: What are the most effective techniques? A Cochrane systematic review. Eur. J. Oral Implantol. 2012, 5, 221–238. [Google Scholar]
- Abrahamsson, I.; Berglundh, T.; Glantz, P.O.; Lindhe, J. The mucosal attachment at different abutments. An experimental study in dogs. J. Clin. Periodontol. 1998, 25, 721–727. [Google Scholar] [CrossRef]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445. [Google Scholar] [CrossRef] [Green Version]
- Zafar, M.S.; Farooq, I.; Awais, M.; Najeeb, S.; Khurshid, Z.; Zohaib, S. Bioactive surface coatings for enhancing osseointegration of dental implants. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–329. [Google Scholar]
- Kim, Y.S.; Shin, S.Y.; Moon, S.K.; Yang, S.M. Surface properties correlated with the human gingival fibroblasts attachment on various materials for implant abutments: A multiple regression analysis. Acta Odontol. Scand. 2015, 73, 38–47. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Eskow, R.N. Preservation of implant esthetics: Soft tissue and restorative considerations. J. Esthet. Dent. 1996, 8, 12–19. [Google Scholar] [CrossRef]
- Ferrari, M.; Cagidiaco, M.C.; Garcia-Godoy, F.; Goracci, C.; Cairo, F. Effect of different prosthetic abutments on peri-implant soft tissue. A randomized controlled clinical trial. Am. J. Dent. 2015, 28, 85–89. [Google Scholar]
- Kim, Y.S.; Ko, Y.; Kye, S.B.; Yang, S.M. Human gingival fibroblast (HGF-1) attachment and proliferation on several abutment materials with various colors. Int. J. Oral Maxillofac. Implants 2014, 29, 969–975. [Google Scholar] [CrossRef] [Green Version]
- Rath, H.; Stumpp, S.N.; Stiesch, M. Development of a flow chamber system for the reproducible in vitro analysis of biofilm formation on implant materials. PLoS ONE 2017, 12, e0172095. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.S.; Costerton, J.W.; Lamont, R.J. Biofilm formation by Porphyromonas gingivalis and Streptococcus gordonii. J. Periodontal Res. 1998, 33, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Rath, H.; Feng, D.; Neuweiler, I.; Stumpp, N.S.; Nackenhorst, U.; Stiesch, M. Biofilm formation by the oral pioneer colonizer Streptococcus gordonii: An experimental and numerical study. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Kolenbrander, P.E. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J. Bacteriol. 2009, 191, 6804–6811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.F.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implants Res. 2006, 17, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Prachar, P.; Bartakova, S.; Brezina, V.; Cvrcek, L.; Vanek, J. Cytocompatibility of implants coated with titanium nitride and zirconium nitride. Bratisl. Lek. Listy 2015, 116, 154–156. [Google Scholar] [CrossRef] [Green Version]
- Brunello, G.; Brun, P.; Gardin, C.; Ferroni, L.; Bressan, E.; Meneghello, R.; Zavan, B.; Sivolella, S. Biocompatibility and antibacterial properties of zirconium nitride coating on titanium abutments: An in vitro study. PLoS ONE 2018, 13, e0199591. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Kondo, R.; Oshiro, W.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Soft tissue sealing around dental implants based on histological interpretation. J. Prosthodont. Res. 2016, 60, 3–11. [Google Scholar] [CrossRef]
- Violant, D.; Galofré, M.; Nart, J.; Teles, R. In vitro evaluation of a multispecies oral biofilm on different implant surfaces. Biomed. Mater. 2014, 9, 035007. [Google Scholar] [CrossRef]
- Geng, H.; O’Neill, M.B.; Kacar, T.; Wilson, B.R.; Oren, E.E.; Sarikaya, M.; Tamerler, C. Engineered chimeric peptides with antimicrobial and titanium-binding functions to inhibit biofilm formation on Ti implants. Mater. Sci. Eng. C 2018, 82, 141–154. [Google Scholar] [CrossRef]
- Badran, Z.; Struillou, X.; Hughes, F.J.; Soueidan, A.; Hoornaert, A.; Ide, M. Silicon Nitride (Si3N4) Implants: The Future of Dental Implantology? J. Oral Implantol. 2017, 43, 240–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Àlvarez, G.; González, M.; Isabal, S.; Blanc, V.; León, R. Method to quantify live and dead cells in multi-species oral biofilm by real-time PCR with propidium monoazide. AMB Express 2013, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. The effects of diode laser on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide adherent to titanium oxide surface of dental implants. An in vitro study. Lasers Med. Sci. 2016, 31, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhurakivska, K.; Ciacci, N.; Troiano, G.; Caponio, V.C.A.; Scrascia, R.; Pallecchi, L.; Lo Muzio, L.; Arena, F. Nitride-Coated and Anodic-Oxidized Titanium Promote a Higher Fibroblast and Reduced Streptococcus gordonii Proliferation Compared to the Uncoated Titanium. Prosthesis 2020, 2, 333-339. https://0-doi-org.brum.beds.ac.uk/10.3390/prosthesis2040031
Zhurakivska K, Ciacci N, Troiano G, Caponio VCA, Scrascia R, Pallecchi L, Lo Muzio L, Arena F. Nitride-Coated and Anodic-Oxidized Titanium Promote a Higher Fibroblast and Reduced Streptococcus gordonii Proliferation Compared to the Uncoated Titanium. Prosthesis. 2020; 2(4):333-339. https://0-doi-org.brum.beds.ac.uk/10.3390/prosthesis2040031
Chicago/Turabian StyleZhurakivska, Khrystyna, Nagaia Ciacci, Giuseppe Troiano, Vito Carlo Alberto Caponio, Roberto Scrascia, Lucia Pallecchi, Lorenzo Lo Muzio, and Fabio Arena. 2020. "Nitride-Coated and Anodic-Oxidized Titanium Promote a Higher Fibroblast and Reduced Streptococcus gordonii Proliferation Compared to the Uncoated Titanium" Prosthesis 2, no. 4: 333-339. https://0-doi-org.brum.beds.ac.uk/10.3390/prosthesis2040031






