A Review of Current and New Optical Techniques for Coral Monitoring
Abstract
:1. Introduction
2. Current Optic Based Methods for Monitoring Coral Health
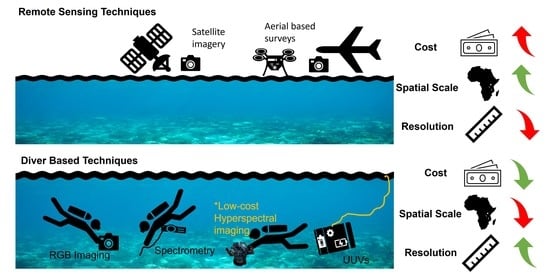
2.1. Diver-Based Survey Techniques
2.1.1. RGB Imaging
2.1.2. Underwater Spectroscopic Techniques
2.1.3. The Potential of ‘New’ Underwater Hyperspectral Imagers
2.2. Remote Sensing Techniques
2.2.1. Airborne Multi/Hyperspectral Imaging
2.2.2. Satellite Multi/Hyperspectral Imaging
2.3. Limitations of Non-Invasive Monitoring
| Model | Developer | Spectral Range/Bands | Resolution | Spatial Imaging | Depth Rating | Cost (£) | Notes |
|---|---|---|---|---|---|---|---|
| Bi-Frost DSLR | Bristol University | 339–789 nm/192 | 18 nm@450 nm | Push-broom | 60 m | ~5000 | |
| TuLUMIS | Liu et al., 2018 [104] | 400–700 nm/8 | >10 nm | Staring array | 2000 m | ~5210.00 ($6730) | UUV mounted |
| HyperDiver | Chennu et al., 2017 [70] | 400–900 nm/480 | 1.5 nm | Push-broom | 50 m | ~20,040 (22,000 €) | Air weight ~32 kg |
| LUMIS 2 | Zawada et al., 2010 [105] | 460,522,582,678 nm/4 | 12.0–42.1 nm | Staring array | 20 m | ~46,470 ($60,000) | 4 imagers used |
| U185 | Cubert Gmbh | 450–950 nm/125 | 8 nm @ 532 nm | Snapshot | 5 m | ~49,850 (54,900 €) | |
| WaterCam | Sphere Optics | 450–950 nm/138 | 8 nm @ 532 nm | Snapshot | 5 m | ~49,850 (54,900 €) | |
| UMSI | Wu et al., 2019 [106] | 400–700 nm/31 | 10 nm | Staring | 50 m | ~56,170 ($72,800) | |
| UHI OV | Ecotone | 380–750 nm/150–200 | 2.2–5.5 nm | Push-broom | 2000 m | ~57,800 ($75,000) | UUV mounted |
| Technique | Cost | Spatial Scale | Spatial Resolution | Additional Data Gathered | Notes |
|---|---|---|---|---|---|
| RGB imaging (Based on GoPro) | Very Low | Moderate | Very High | Photogrammetry | Limited spectral data obtained |
| Spectrometers (Waltz Diving PAM) | Moderate | Very Low | Very High | N/A | N/A |
| Bi-Frost DSLR | Low | Moderate | Very High | Photogrammetry, Fluorescence (HyFi) | Night-time imaging required for Fluorescence |
| Current UHI systems (See Table 4) | Moderate to High | Moderate | Very High to Moderate | N/A | Often large and cumbersome or designed specifically for UUVs |
| Drone multi/hyperspectral imaging [81] | Moderate to High | Moderate | Moderate | N/A | Requires Ground truthing |
| Aeroplane multi/hyperspectral imaging [61,107] | High | High | Moderate to Low | N/A | Requires Ground truthing |
| Satellite multi/hyperspectral imaging [108] | * Very High | Very High | Low to Very Low | SST, RGB images (Dependant on additional sensors equipped) | Requires Ground truthing |
| Classification | Cost | Spatial Scale | Spatial Resolution |
|---|---|---|---|
| Very Low | <£1000 | mm–cm | km |
| Low | <£5000 | m | <100 m |
| Moderate | <£10,000 | <100 m | <10 m |
| High | >£25,000 | km | m |
| Very High | >£100,000 | 100+ km | mm-cm |
| Criteria | RGB Imaging | Spectrometers | Bi-Frost DSLR | Drone Multi/Hyperspectral Imaging | Aeroplane Multi/Hyperspectral Imaging | Satellite Multi/Hyperspectral Imaging |
|---|---|---|---|---|---|---|
| Damage (Disease/Bleaching) | Yes | Yes (Local Scale) | Yes | Yes | Yes (Mass Scale) | Yes (Mass Scale) |
| Recruits | Yes (modified camera) | No | Yes (HyFi) | No | No | No |
| Number of Colonies | Yes | No | Yes | Yes | Yes (Height Dependent) | No |
| Growth Measurements | Yes | No | Yes | No | No | No |
| Repeatability * | No | No | Yes (Reef Scale) | Yes | Yes | Yes |
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- Douglas, A.E. Coral bleaching—How and why? Mar. Pollut. Bull. 2003, 46, 385–392. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R.; Larkum, A. Zooxanthellae expelled from bleached corals at 33 °C are photosynthetically competent. Mar. Ecol. Prog. Ser. 2001, 220, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Willis, B.L.; Page, C.A.; Dinsdale, E.A. Coral Disease on the Great Barrier Reef. Coral Health Dis. 2002, 69–104. [Google Scholar] [CrossRef]
- Sutherland, K.P.; Porter, J.W.; Torres, C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 2004, 266, 273–302. [Google Scholar] [CrossRef] [Green Version]
- Zaneveld, J.R.; Burkepile, D.E.; Shantz, A.; Pritchard, C.E.; McMinds, R.; Payet, J.P.; Welsh, R.; Correa, A.M.S.; Lemoine, N.P.; Rosales, S.; et al. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat. Commun. 2016, 7, 11833. [Google Scholar] [CrossRef]
- Mumby, P.J. The Impact of Exploiting Grazers (Scaridae) on the Dynamics of Caribbean Coral Reefs. Ecol. Appl. 2006, 16, 747–769. [Google Scholar] [CrossRef] [Green Version]
- Mora, C. A clear human footprint in the coral reefs of the Caribbean. Proc. R. Soc. B Boil. Sci. 2008, 275, 767–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenger, A.S.; Fabricius, K.E.; Jones, G.P.; Brodie, J.E. Effects of sedimentation, eutrophication, and chemical pollution on coral reef fishes. In Ecology of Fishes on Coral Reefs; Mora, C., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 145–153. [Google Scholar]
- Kayal, M.; Vercelloni, J.; De Loma, T.L.; Bosserelle, P.; Chancerelle, Y.; Geoffroy, S.; Stievenart, C.; Michonneau, F.; Penin, L.; Planes, S.; et al. Predator Crown-of-Thorns Starfish (Acanthaster planci) Outbreak, Mass Mortality of Corals, and Cascading Effects on Reef Fish and Benthic Communities. PLoS ONE 2012, 7, e47363. [Google Scholar] [CrossRef] [Green Version]
- Bak, R.P.M.; van Eys, G. Predation of the sea urchin Diadema antillarum Philippi on living coral. Oecologia 1975, 20, 111–115. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef] [Green Version]
- Hoegh-Guldberg, O.; Pendleton, L.; Kaup, A. People and the changing nature of coral reefs. In Regional Studies in Marine Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 30, p. 100699. [Google Scholar] [CrossRef]
- Lamb, J.B.; True, J.D.; Piromvaragorn, S.; Willis, B.L. Scuba diving damage and intensity of tourist activities increases coral disease prevalence. Biol. Conserv. 2014, 178, 88–96. [Google Scholar] [CrossRef]
- Roth, M.S.; Deheyn, D.D. Effects of cold stress and heat stress on coral fluorescence in reef-building corals. Sci. Rep. 2013, 3, 1421. [Google Scholar] [CrossRef] [Green Version]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glynn, P.W. Coral reef bleaching: Ecological perspectives. Coral Reefs 1993, 12, 1–17. [Google Scholar] [CrossRef]
- Rogers, C.S. The effect of shading on coral reef structure and function. J. Exp. Mar. Biol. Ecol. 1979, 41, 269–288. [Google Scholar] [CrossRef]
- Sheppard, C.; Davy, S.; Pilling, G.; Graham, N. The Biology of Coral Reefs; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Field, S.F.; Bulina, M.Y.; Kelmanson, I.V.; Bielawski, J.P.; Matz, M.V. Adaptive Evolution of Multicolored Fluorescent Proteins in Reef-Building Corals. J. Mol. Evol. 2006, 62, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Antonius, A. The ‘band’ diseases in coral reefs. In Proceedings of the Fourth International Coral Reef Symposium, Manila, Philippines, 18–22 May 1981. [Google Scholar]
- Galloway, S.B.; Bruckner, A.W.; Woodley, C.M. The Global Perspective on Incidence and Prevalence of Coral Diseases; NOAA Technical Memorandum NOS NCCOS 97 and CRCP 7; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2009. [Google Scholar]
- Ruiz-Moreno, D.; Willis, B.L.; Page, A.C.; Weil, E.; Croquer, A.; Vargas-Angel, B.; Jordan-Garza, A.G.; Jordán-Dahlgren, E.; Raymundo, L.; Harvell, C.D. Global coral disease prevalence associated with sea temperature anomalies and local factors. Dis. Aquat. Org. 2012, 100, 249–261. [Google Scholar] [CrossRef] [Green Version]
- Dinsdale, A.E. Abundance of black-band disease on corals from one location on the Great Barrier Reef: A comparison with abundance in the Caribbean region. In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000. [Google Scholar]
- Miller, I. Black band disease on the Great Barrier Reef. Coral Reefs 1996, 15, 58. [Google Scholar]
- Cervino, J.M.; Thompson, F.L.; Gomez-Gil, B.; Lorence, E.A.; Goreau, T.J.; Hayes, R.L.; Winiarski-Cervino, K.B.; Smith, G.W.; Hughen, K.; Bartels, E.; et al. The Vibrio core group induces yellow band disease in Caribbean and Indo-Pacific reef-building corals. J. Appl. Microbiol. 2008, 105, 1658–1671. [Google Scholar] [CrossRef]
- Bongiorni, L.; Rinkevich, B. The pink-blue spot syndrome in Acropora eurystoma (Eilat, Red Sea): A possible marker of stress? Zoology 2005, 108, 247–256. [Google Scholar] [CrossRef]
- Ravindran, J.; Raghukumar, C. Pink-line syndrome, a physiological crisis in the scleractinian coral Porites lutea. Mar. Biol. 2006, 149, 347–356. [Google Scholar] [CrossRef]
- Antonius, A. Black band disease infection experiments on hexacorals and octocorals. In Proceedings of the Fifth International Coral Reef Congress, Tahiti, French, 27 May–1 June 1985. [Google Scholar]
- Green, E.P.; Bruckner, A.W. The significance of coral disease epizootiology for coral reef conservation. Biol. Conserv. 2000, 96, 347–361. [Google Scholar] [CrossRef]
- Palmer, C.V.; Modi, C.K.; Mydlarz, L.D. Coral Fluorescent Proteins as Antioxidants. PLoS ONE 2009, 4, e7298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, E.M.; Van Woesik, R. Caribbean coral diseases: Primary transmission or secondary infection? Glob. Chang. Biol. 2012, 18, 3529–3535. [Google Scholar] [CrossRef]
- Teague, J.; Willans, J.; Allen, M.; Scott, T.; Day, J. Hyperspectral imaging as a tool for assessing coral health utilising natural fluorescence. J. Spectr. Imaging 2019, 8, a7. [Google Scholar] [CrossRef] [Green Version]
- Holden, H.; LeDrew, E. Spectral Discrimination of Healthy and Non-Healthy Corals Based on Cluster Analysis, Principal Components Analysis, and Derivative Spectroscopy. Remote Sens. Environ. 1998, 65, 217–224. [Google Scholar] [CrossRef]
- Myers, M.R.; Hardy, J.T.; Mazel, C.H.; Dustan, P. Optical spectra and pigmentation of Caribbean reef corals and macroalgae. Coral Reefs 1999, 18, 179–186. [Google Scholar] [CrossRef]
- Leiper, I.A.; Siebeck, U.E.; Marshall, N.J.; Phinn, S.R. Coral health monitoring: Linking coral colour and remote sensing techniques. Can. J. Remote Sens. 2009, 35, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.S. Absorption and fluorescence spectra of chlorophyll-proteins isolated from Euglena gracilis. Biochim. Biophys. ActaBioenergy 1980, 591, 9–21. [Google Scholar] [CrossRef]
- Falkowski, P.; Knoll, A.H. Evolution of Primary Producers in the Sea; Academic Press; Elsevier: New York, NY, USA, 2007. [Google Scholar]
- Alieva, N.O.; Konzen, K.A.; Field, S.F.; Meleshkevitch, E.A.; Hunt, M.E.; Beltran-Ramirez, V.; Miller, D.J.; Wiedenmann, J.; Salih, A.; Matz, M.V. Diversity and Evolution of Coral Fluorescent Proteins. PLoS ONE 2008, 3, e2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, T.S.; Corredor, J. Studies of photosynthetic pigments of zooxanthellae in Caribbean hermatypic corals. In Proceedings of the Fourth International Coral Reefs Symposium, Manila, Philippines, 18–22 May 1981. [Google Scholar]
- Venn, A.A.; Wilson, M.A.; Trapido-Rosenthal, H.G.; Keely, B.J.; Douglas, A.E. The impact of coral bleaching on the pigment profile of the symbiotic alga, Symbiodinium. Plant Cell Environ. 2006, 29, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lanetty, M.; Harii, S.; Hoegh-Guldberg, O. Early molecular responses of coral larvae to hyperthermal stress. Mol. Ecol. 2009, 18, 5101–5114. [Google Scholar] [CrossRef]
- Desalvo, M.K.; Voolstra, C.R.; Sunagawa, S.; Schwarz, J.A.; Stillman, J.H.; Coffroth, M.A.; Szmant, A.M.; Medina, M. Differential gene expression during thermal stress and bleaching in the Caribbean coralMontastraea faveolata. Mol. Ecol. 2008, 17, 3952–3971. [Google Scholar] [CrossRef]
- Hill, J.; Wilkinson, C. Methods for Ecological Monitoring of Coral Reefs; Australian Institute of Marine Science: Townsville, Australia, 2004.
- Bruno, J.F.; Selig, E.R. Regional Decline of Coral Cover in the Indo-Pacific: Timing, Extent, and Subregional Comparisons. PLoS ONE 2007, 2, e711. [Google Scholar] [CrossRef] [Green Version]
- Page, C.A.; Baker, D.M.; Harvell, C.D.; Golbuu, Y.; Raymundo, L.; Neale, S.J.; Rosell, K.B.; Rypien, K.L.; Andras, J.P.; Willis, B.L. Influence of marine reserves on coral disease prevalence. Dis. Aquat. Organ. 2009, 87, 135–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, C.A.; Field, S.N.; Pollock, F.J.; Lamb, J.; Shedrawi, G.; Wilson, S.K. Assessing coral health and disease from digital photographs and in situ surveys. Environ. Monit. Assess. 2016, 189, 18. [Google Scholar] [CrossRef]
- Raymundo, L.J.; Couch, C.S.; Bruckner, A.W.; Harvell, C.D. Coral Disease Handbook: Guidelines for Assessment, Monitoring & Management; Coral Reef Targeted Research & Capacity Building for Management (CRTR); Currie Communications: Melbourne, Australia, 2008. [Google Scholar]
- Patterson, M.R.; Relles, N.J. Autonomous Underwater Vehicles resurvey Bonaire: A new tool for coral reef management. In Proceedings of the 11th International Coral Reef Symposium, Fort Lauderdale, FL, USA, 7–11 July 2008. [Google Scholar]
- Williams, S.B.; Pizarro, O.; Jakuba, M.V.; Johnson, C.; Barrett, N.; Babcock, R.; Kendrick, G.; Steinberg, P.D.; Heyward, A.J.; Doherty, P.; et al. Monitoring of Benthic Reference Sites: Using an Autonomous Underwater Vehicle. IEEE Robot. Autom. Mag. 2012, 19, 73–84. [Google Scholar] [CrossRef]
- Bokolonga, E.; Hauhana, M.; Rollings, N.; Aitchison, D.; Assaf, M.; Das, S.R.; Biswas, S.N.; Groza, V.; Petriu, E.M. A compact multispectral image capture unit for deployment on drones. In Proceedings of the Conference Record-IEEE Instrumentation and Measurement Technology Conference, Taipei, Taiwan, 23–26 May 2016. [Google Scholar]
- Beijbom, O.; Edmunds, P.J.; Roelfsema, C.; Smith, J.; Kline, D.I.; Neal, B.P.; Dunlap, M.J.; Moriarty, V.; Fan, T.Y.; Tan, C.J.; et al. Towards Automated Annotation of Benthic Survey Images: Variability of Human Experts and Operational Modes of Automation. PLoS ONE 2015, 10, e0130312. [Google Scholar] [CrossRef]
- Williams, I.D.; Couch, C.; Beijbom, O.; Oliver, T.; Vargas-Angel, B.; Schumacher, B.; Brainard, R. Leveraging Automated Image Analysis Tools to Transform Our Capacity to Assess Status and Trends of Coral Reefs. Front. Mar. Sci. 2019, 6, 222. [Google Scholar] [CrossRef] [Green Version]
- Siebeck, U.E.; Marshall, N.J.; Klüter, A.; Hoegh-Guldberg, O. Monitoring coral bleaching using a colour reference card. Coral Reefs 2006, 25, 453–460. [Google Scholar] [CrossRef]
- Winters, G.; Holzman, R.; Blekhman, A.; Beer, S.; Loya, Y. Photographic assessment of coral chlorophyll contents: Implications for ecophysiological studies and coral monitoring. J. Exp. Mar. Bio. Ecol. 2009, 380, 25–35. [Google Scholar] [CrossRef]
- Fitt, W.K.; McFarland, F.K.; Warner, M.E.; Chilcoat, G.C. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 2000, 45, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Teague, J.; Scott, T.B. Underwater Photogrammetry and 3D Reconstruction of Submerged Objects in Shallow Environments by ROV and Underwater GPS. J. Mar. Sci. Res. Technol. 2017, 1, 1. [Google Scholar]
- Colomina, I.; Molina, P. Unmanned aerial systems for photogrammetry and remote sensing: A review. ISPRS J. Photogramm. Remote Sens. 2014, 92, 79–97. [Google Scholar] [CrossRef] [Green Version]
- Bayley, D.T.I.; Mogg, A.O.M. A protocol for the large-scale analysis of reefs using Structure from Motion photogrammetry. Methods Ecol. Evol. 2020, 11, 1410–1420. [Google Scholar] [CrossRef]
- Hedley, J.D.; Mumby, P.J. Biological and remote sensing perspectives of pigmentation in coral reef organisms. Adv. Mar. Biol. 2002, 43, 277–317. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Atkinson, M.J. Spectral discrimination of coral reef benthic communities. Coral Reefs 2000, 19, 164–171. [Google Scholar] [CrossRef]
- Jones, R.J.; Kildea, T.; Hoegh-Guldberg, O. PAM Chlorophyll Fluorometry: A New in situ Technique for Stress Assessment in Scleractinian Corals, used to Examine the Effects of Cyanide from Cyanide Fishing. Mar. Pollut. Bull. 1999, 38, 864–874. [Google Scholar] [CrossRef]
- Chauka, L.J.; Steinert, G.; Mtolera, M.S.P. Influence of local environmental conditions and bleaching histories on the diversity and distribution of Symbiodinium in reef-building corals in Tanzania. Afr. J. Mar. Sci. 2016, 63, 57–64. [Google Scholar] [CrossRef]
- Kurihara, H.; Takahashi, A.; Reyes-Bermudez, A.; Hidaka, M. Intraspecific variation in the response of the scleractinian coral Acropora digitifera to ocean acidification. Mar. Biol. 2018, 165, 38. [Google Scholar] [CrossRef]
- Chang, C.I. Hyperspectral Imaging: Techniques for Spectral Detection and Classification; Springer Science & Business Media: Berlin, Germany, 2003. [Google Scholar]
- Zawada, D.G. The Application of a Novel Multispectral Imaging System to the in vivo Study of Flourescent Compounds in Selected Marine Organisms. Ph.D. Thesis, University of California, San Diego, CA, USA, 2002. [Google Scholar]
- Gleason, A.C.R.; Reid, R.P.; Voss, K.J. Automated classification of underwater multispectral imagery for coral reef monitoring. In Proceedings of the Oceans Conference Record (IEEE), Charleston, WV, USA, 29 September–4 October 2007. [Google Scholar]
- Sture, O.; Ludvigsen, M.; Soreide, F.; Aas, L.M.S. Autonomous underwater vehicles as a platform for underwater hyperspectral imaging. In Proceedings of the OCEANS 2017, Aberdeen, UK, 19–22 June 2017. [Google Scholar]
- Liu, B.; Liu, Z.; Men, S.; Li, Y.; Ding, Z.; He, J.; Zhao, Z. Underwater Hyperspectral Imaging Technology and Its Applications for Detecting and Mapping the Seafloor: A Review. Sensors 2020, 20, 4962. [Google Scholar] [CrossRef]
- Chennu, A.; Färber, P.; De’Ath, G.; De Beer, D.; Fabricius, K.E. A diver-operated hyperspectral imaging and topographic surveying system for automated mapping of benthic habitats. Sci. Rep. 2017, 7, 7122. [Google Scholar] [CrossRef] [PubMed]
- Maglione, P. Very High Resolution Optical Satellites: An Overview of the Most Commonly used. Am. J. Appl. Sci. 2016, 13, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, G.; Ludvigsen, M.; Sørensen, A.; Sandvik Aas, L.M. The use of underwater hyperspectral imaging deployed on remotely operated vehicles—methods and applications. IFAC-Papers 2016, 49, 476–481. [Google Scholar] [CrossRef]
- Johnsen, G.; Volent, Z.; Dierssen, H.; Pettersen, R.; Ardelan, M.; Søreide, F.; Fearns, P.; Ludvigsen, M.; Moline, M. Underwater hyperspectral imagery to create biogeochemical maps of seafloor properties. In Subsea Optics and Imaging; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Pust, O. Innovative Filter Solutions for Hyperspectral Imaging. Opt. Photonik 2016, 11, 24–27. [Google Scholar] [CrossRef]
- Renhorn, I.G.E.; Bergström, D.; Hedborg, J.; Letalick, D.; Möller, S. High spatial resolution hyperspectral camera based on a linear variable filter. Opt. Eng. 2016, 55, 114105. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Gibson, D.; Ahmadzadeh, S.; Chu, H.O.; Warden, B.; Overend, R.; Macfarlane, F.; Murray, P.; Marshall, S.; Aitkenhead, M.; et al. Low-cost hyper-spectral imaging system using a linear variable bandpass filter for agritech applications. Appl. Opt. 2020, 59, A167–A175. [Google Scholar] [CrossRef]
- Burns, J.; Delparte, D.M.; Gates, R.D.; Takabayashi, M. Integrating structure-from-motion photogrammetry with geospatial software as a novel technique for quantifying 3D ecological characteristics of coral reefs. PeerJ 2015, 3, e1077. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.R.; Hochberg, E.; Asner, G.P.; Green, R.O.; Knapp, D.E.; Gao, B.-C.; Garcia, R.; Gierach, M.; Lee, Z.; Maritorena, S.; et al. Airborne mapping of benthic reflectance spectra with Bayesian linear mixtures. Remote Sens. Environ. 2017, 200, 18–30. [Google Scholar] [CrossRef]
- Berkelmans, R.; De’Ath, G.; Kininmonth, S.; Skirving, W.J.S. A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: Spatial correlation, patterns, and predictions. Coral Reefs 2004, 23, 74–83. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Boyd, J. Drones survey the great barrier reef: Aided by AI, hyperspectral cameras can distinguish bleached from unbleached coral-[News]. IEEE Spectr. 2019, 56, 7–9. [Google Scholar] [CrossRef]
- Queensland University of Technology (QUT). Queensland’s Own Rapid Response Tool for Monitoring Coral Bleaching. 2017. Available online: https://www.qut.edu.au/news?news-id=122198 (accessed on 12 June 2018).
- Sully, S.; Burkepile, D.E.; Donovan, M.K.; Hodgson, G.; Van Woesik, R. A global analysis of coral bleaching over the past two decades. Nat. Commun. 2019, 10, 1264. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.E.; Dunne, R.P.; Ambarsari, I.; Le Tissier, M.D.A.; Satapoomin, U. Seasonal fluctuations in environmental factors and variations in symbiotic algae and chlorophyll pigments in four Indo-Pacific coral species. Mar. Ecol. Prog. Ser. 1999, 191, 53–69. [Google Scholar] [CrossRef] [Green Version]
- Strong, A.E.; Barrientos, C.S.; Duda, C.; Sapper, J. Improved satellite techniques for monitoring coral reef bleaching. In Proceedings of the 8th International Coral Reef Symposium, Panama City, Panama, 24–29 June 1996. [Google Scholar]
- Liu, G.; Strong, A.E.; Skirving, W.J.; Arzayus, L.F. Overview of NOAA Coral Reef Watch Program’s Near-Real-Time Satellite Global Coral Bleaching Monitoring Activities. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2004. [Google Scholar]
- Liu, G.; Heron, S.F.; Eakin, C.M.; Muller-Karger, F.E.; Vega-Rodriguez, M.; Guild, L.S.; De La Cour, J.L.; Geiger, E.F.; Skirving, W.J.; Burgess, T.F.R.; et al. Reef-Scale Thermal Stress Monitoring of Coral Ecosystems: New 5-km Global Products from NOAA Coral Reef Watch. Remote Sens. 2014, 6, 11579–11606. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.L. Comparison of simulated hyperspectral HyspIRI and multispectral Landsat 8 and Sentinel-2 imagery for multi-seasonal, regional land-cover mapping. Remote Sens. Environ. 2017, 200, 311–325. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Joyce, K.E.; Phinn, S.R.; Roelfsema, C.M.; Neil, D.T.; Dennison, W.C. Combining Landsat ETM+ and Reef Check classifications for mapping coral reefs: A critical assessment from the southern Great Barrier Reef, Australia. Coral Reefs 2004, 23, 21–25. [Google Scholar] [CrossRef]
- Palandro, D.; Andréfouët, S.; Muller-Karger, F.E.; Dustan, P.; Hu, C.; Hallock, P. Detection of changes in coral reef communities using Landsat-5 TM and Landsat-7 ETM+ data. Can. J. Remote Sens. 2003, 29, 201–209. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Y.; Li, M.; Zhou, M.; Yang, Y. Survey of reefs based on Landsat 8 operational land imager (OLI) images in the Nansha Islands, South China Sea. Acta Oceanol. Sin. 2016, 35, 11–19. [Google Scholar] [CrossRef]
- Keith, D.J.; Schaeffer, B.A.; Lunetta, R.S.; Gould, R.W., Jr.; Rocha, K.; Cobb, D.J. Remote sensing of selected water-quality indicators with the hyperspectral imager for the coastal ocean (HICO) sensor. Int. J. Remote Sens. 2014, 35, 2927–2962. [Google Scholar] [CrossRef]
- O. S. University. What Is HICO? 2015. Available online: http://hico.coas.oregonstate.edu/ (accessed on 12 June 2018).
- Stumpf, R.P.; Holderied, K.; Sinclair, M. Determination of water depth with high-resolution satellite imagery over variable bottom types. Limnol. Oceanogr. 2003, 48, 547–556. [Google Scholar] [CrossRef]
- Foo, S.A.; Asner, G.P. Scaling Up Coral Reef Restoration Using Remote Sensing Technology. Front. Mar. Sci. 2019, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- Casella, E.; Collin, A.; Harris, D.; Ferse, S.; Bejarano, S.; Parravicini, V.; Hench, J.L.; Rovere, A. Mapping coral reefs using consumer-grade drones and structure from motion photogrammetry techniques. Coral Reefs 2017, 36, 269–275. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Peltier, S.A.; Maritorena, S. Trends and variability in spectral diffuse attenuation of coral reef waters. Coral Reefs 2020, 39, 1377–1389. [Google Scholar] [CrossRef]
- Takabayashi, M.; Hoegh-Guldberg, O. Ecological and physiological differences between two colour morphs of the coral Pocillopora damicornis. Mar. Biol. 1995, 123, 705–714. [Google Scholar] [CrossRef]
- Anderson, D.A.; Armstrong, R.A.; Weil, E. Hyperspectral Sensing of Disease Stress in the Caribbean Reef-Building Coral, Orbicella faveolate—Perspectives for the Field of Coral Disease Monitoring. PLoS ONE 2013, 8, e81478. [Google Scholar] [CrossRef]
- Johannes, R.E.; Wiebe, W.J. Method for Derterminations of Coral Tissue Biomass and Composition. Limnol. Oceanogr. 1970, 15, 822–824. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Rodgers, K.S.; Brown, E.K.; Kenyon, J.C.; Aeby, G.; Smith, W.R.; Farrell, F. Comparison of methods used to estimate coral cover in the Hawaiian Islands. PeerJ 2015, 3, e954. [Google Scholar] [CrossRef] [Green Version]
- Leujak, W.; Ormond, R.F.G. Comparative accuracy and efficiency of six coral community survey methods. J. Exp. Mar. Biol. Ecol. 2007, 351, 168–187. [Google Scholar] [CrossRef]
- Liu, H.; Sticklus, J.; Köser, K.; Hoving, H.-J.T.; Song, H.; Chen, Y.; Greinert, J.; Schoening, T. TuLUMIS—A tunable LED-based underwater multispectral imaging system. Opt. Express 2018, 26, 7811–7828. [Google Scholar] [CrossRef] [PubMed]
- Zawada, D.G.; Jaffe, J.S. Changes in the fluorescence of the Caribbean coral Montastraea faveolata during heat-induced bleaching. Limnol. Oceanogr. 2010, 48, 412–425. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Shentu, Y.; Chaofan, C.; Guo, Y.; Zhang, Y.; Wei, H.; Yang, P.; Huang, H.; Song, H. Development of an underwater multispectral imaging system based on narrowband color filters. In Proceedings of the OCEANS 2018 MTS/IEEE, Charleston, SC, USA, 22–25 October 2019. [Google Scholar]
- Kobryn, H.T.; Wouters, K.; Beckley, L.E.; Heege, T. Ningaloo Reef: Shallow Marine Habitats Mapped Using a Hyperspectral Sensor. PLoS ONE 2013, 8, e70105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedley, J.D.; Roelfsema, C.M.; Chollett, I.; Harborne, A.R.; Heron, S.F.; Weeks, S.; Skirving, W.J.; Strong, A.E.; Eakin, C.M.; Christensen, T.R.L.; et al. Remote Sensing of Coral Reefs for Monitoring and Management: A Review. Remote Sens. 2016, 8, 118. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teague, J.; Megson-Smith, D.A.; Allen, M.J.; Day, J.C.C.; Scott, T.B. A Review of Current and New Optical Techniques for Coral Monitoring. Oceans 2022, 3, 30-45. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans3010003
Teague J, Megson-Smith DA, Allen MJ, Day JCC, Scott TB. A Review of Current and New Optical Techniques for Coral Monitoring. Oceans. 2022; 3(1):30-45. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans3010003
Chicago/Turabian StyleTeague, Jonathan, David A. Megson-Smith, Michael J. Allen, John C.C. Day, and Thomas B. Scott. 2022. "A Review of Current and New Optical Techniques for Coral Monitoring" Oceans 3, no. 1: 30-45. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans3010003