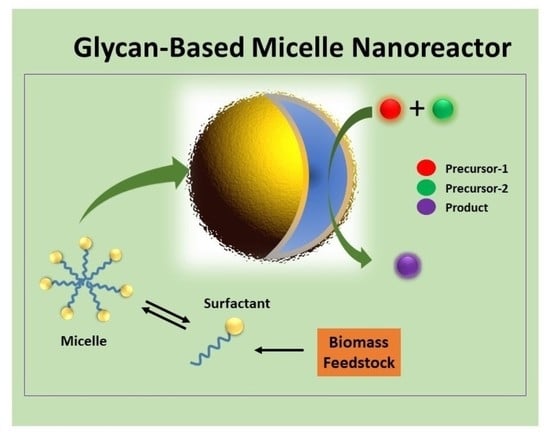
A Review on Recent Progress of Glycan-Based Surfactant Micelles as Nanoreactor Systems for Chemical Synthesis Applications
Abstract
:1. Introduction
1.1. Types of Glycan-Based Surfactants
1.1.1. Span and Tween
1.1.2. Alkyl Polyglycosides
1.1.3. Fatty Acid Glucamides
1.1.4. Sucrose Esters
1.2. Micellization Properties
1.2.1. Surface Tension Measurements
1.2.2. Particle Size Distribution of Micelles
1.2.3. Adsorption Behaviour
2. Application of Glycan-Based Surfactant’s Nanoreactors for Chemical Synthesis
2.1. Synthesis of Inorganic Compounds
2.2. Polymerization
2.3. Synthesis of Organic Compounds
2.4. Other Applications
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, M.; Singh, B. A Brief Review of Construction, Working and Applications of Nanoreactors. Chem. Sci. J. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, J.; Zhang, X. Nanoreactors for Chemical Synthesis and Biomedical Applications. Chem. Asian J. 2019, 14, 3240–3250. [Google Scholar] [CrossRef] [PubMed]
- Zetterlund, P.B. Controlled/living radical polymerization in nanoreactors: Compartmentalization effects. Polym. Chem. 2011, 2, 534–549. [Google Scholar] [CrossRef]
- Abe, H.; Mawatari, Y.; Teraoka, H.; Fujimoto, K.; Inouye, M. Synthesis and Molecular Recognition of Pyrenophanes with Polycationic or Amphiphilic Functionalities: Artificial Plate-Shaped Cavitant Incorporating Arenes and Nucleotides in Water. J. Org. Chem. 2004, 69, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Romney, D.K.; Arnold, F.H.; Lipshutz, B.H. Chemistry Takes a Bath: Reactions in Aqueous Media. J. Org. Chem. 2018, 83, 7319–7322. [Google Scholar] [CrossRef] [Green Version]
- Sigman, R.; Ladeuille, B. OECD Environmental Outlook for the Chemicals Industry; OECD: Paris, France, 2001. [Google Scholar]
- Cui, X.; Li, B.; Liu, T.; Li, C. A Practical solution for aqueous reactions of water-insoluble high-melting-point organic substrates. Green Chem. 2012, 14, 668–672. [Google Scholar] [CrossRef]
- Zuo, Y.; Qu, J. How Does Aqueous Solubility of Organic Reactant Affect a Water-Promoted reaction? J. Org. Chem. 2014, 79, 6832–6839. [Google Scholar] [CrossRef]
- Bai, C.C.; Tian, B.R.; Zhao, T.; Huang, Q.; Wang, Z.Z. Cyclodextrin-Catalyzed Organic Synthesis: Reactions, Mechanisms, and Applications. Molecules 2017, 22, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, M.D.; Manna, P.L.; Talotta, C.; Soriente, A. Supramolecular Organocatalysis in Water Mediated by Macrocyclic Compounds. Front. Chem. 2018, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Grommet, A.B.; Feller, M.; Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 2020, 15, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Vriezema, D.M.; Aragonès, M.C.; Elemans, J.A.A.W.; Cornelissen, J.J.L.M.; Rowan, A.E.; Nolte, R.J.M. Self-assembled nanoreactors. Chem. Rev. 2005, 105, 1445–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, H.; Weitzel, H.P.; Huster, W. Aqueous Emulsion Polymers. In Polymer Science: A Comprehensive Reference; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 10, ISBN 9780080878621. [Google Scholar]
- Anderson, C.D.; Daniels, E.S.; Joong, P.; Chang, Y.; Han, D. Emulsion Polymerisation and Applications of Latex; Rapra Technology Limited: Shrewsbury, UK, 2000; ISBN 1-85957-381-9. [Google Scholar]
- Lian, X.; Huang, Y.; Zhu, Y.; Fang, Y.; Zhao, R.; Joseph, E.; Li, J.; Pellois, J.P.; Zhou, H.C. Enzyme-MOF Nanoreactor Activates Nontoxic Paracetamol for Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 5725–5730. [Google Scholar] [CrossRef]
- Anderson, S.L.; Boyd, P.G.; Gładysiak, A.; Nguyen, T.N.; Palgrave, R.G.; Kubicki, D.; Emsley, L.; Bradshaw, D.; Rosseinsky, M.J.; Smit, B.; et al. Nucleobase pairing and photodimerization in a biologically derived metal-organic framework nanoreactor. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Waghmode, B.J.; Bhange, S.N.; Unni, S.M.; Patil, K.R.; Malkhede, D.D. In situ grown nickel nanoparticles in a calixarene nanoreactor on a graphene-MoS2 support for efficient water electrolysis. Sustain. Energy Fuels 2017, 1, 1329–1338. [Google Scholar] [CrossRef]
- Guo, Y.; Solovyov, A.; Grosso-Giordano, N.A.; Hwang, S.J.; Katz, A. Stabilizing Single Sites on Solid Supports: Robust Grafted Ti(IV)-Calixarene Olefin Epoxidation Catalysts via Surface Polymerization and Cross-Linking. ACS Catal. 2016, 6, 7760–7768. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, Z.-J.; Hill, E.H.; Zheng, Y.; Guo, G.; Wang, Y.; Weiss, P.S.; Yu, J.; Yang, Y.-W. Organic-Inorganic Hybrid Pillarene-Based Nanomaterial for Label-Free Sensing and Catalysis. Matter 2019, 1, 848–861. [Google Scholar] [CrossRef] [Green Version]
- Du, X.S.; Jia, Q.; Wang, C.Y.; Meguellati, K.; Yang, Y.W. A pillar[5]arene with an amino-terminated arm stabilizes the formation of aliphatic hemiaminals and imines. Chem. Commun. 2019, 55, 5736–5739. [Google Scholar] [CrossRef]
- Ramazani, A.; Ahmadi, Y.; Aghahosseini, H.; Joo, S.W. “β-Cyclodextrin nano-reactor”-catalyzed synthesis of 2H-chromene-2,3-dicarboxylates from in-situ-generated stabilized phosphorus ylides via intramolecular Wittig reaction in water. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 354–358. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Liu, Q.; Chen, H.; Liu, Y.; Zhou, Y. A novel hollow-sphere cyclodextrin nanoreactor for the enhanced removal of bisphenol A under visible irradiation. J. Hazard. Mater. 2020, 384, 121267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Sonzini, S.; Ambarwati, M.; Rosta, E.; Scherman, O.A.; Herrmann, A. Turning Cucurbit[8]uril into a Supramolecular Nanoreactor for Asymmetric Catalysis. Angew. Chem. Int. Ed. 2015, 54, 13007–13011. [Google Scholar] [CrossRef] [Green Version]
- Lagona, J.; Fettinger, J.C.; Isaacs, L. Cucurbit[n]uril analogues. Org. Lett. 2003, 5, 3745–3747. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Cao, C.; Liu, J.; Wang, X.; Zhu, Y.; Song, W. One methyl group makes a major difference: Shape-selective catalysis by zeolite nanoreactors in liquid-phase condensation reactions. J. Mater. Chem. A 2017, 5, 17464–17469. [Google Scholar] [CrossRef]
- Lee, J.H.; Bonte, W.; Corthals, S.; Krumeich, F.; Ruitenbeek, M.; Van Bokhoven, J.A. Zeolite Nanoreactor for Investigating Sintering Effects of Cobalt-Catalyzed Fischer-Tropsch Synthesis. Ind. Eng. Chem. Res. 2019, 58, 5140–5145. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Y. Synthesis of nanocrystalline LaFeO3 powders via glucose sol–gel route. Mater. Chem. Phys. 2011, 129, 1047–1050. [Google Scholar] [CrossRef]
- Talebian, N.; Kheiri, M. Sol–gel derived nanostructured nickel oxide films: Effect of solvent on crystallographic orientations. Solid State Sci. 2014, 27, 79–83. [Google Scholar] [CrossRef]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. Surfactants and their applications. Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 2003, 99, 3–48. [Google Scholar] [CrossRef]
- Bland, A.S. Global Surfactants Industry. Available online: https://ihsmarkit.com/research-analysis/global-surfactants-industry.html (accessed on 3 March 2021).
- Petrosko, S.H.; Johnson, R.; White, H.; Mirkin, C.A. Nanoreactors: Small Spaces, Big Implications in Chemistry. J. Am. Chem. Soc. 2016, 138, 7443–7445. [Google Scholar] [CrossRef] [Green Version]
- Mańko, D.; Zdziennicka, A. Sugar-based surfactants as alternative to synthetic ones. Ann. Univ. Mariae Curie-Sklodowska Sect. AA Chem. 2015, 70, 161–168. [Google Scholar] [CrossRef]
- Hill, K.; LeHen-Ferrenbach, C. Sugar-Based Surfactants: Fundamentals and Applications, 1st ed.; Ruiz, C.C., Ed.; CRC Taylor and Francis: New York, NY, USA, 2008; ISBN 9780367386245. [Google Scholar]
- Hayes, G.D.; Smith, G. Biobased Surfactants: Overview and Industrial State of the Art; Hayes, D.G., Solaiman, D.K.Y., Ashby, R.D., Eds.; AOCS Press: Urbana, IL, USA, 2019; ISBN 9780128127056. [Google Scholar]
- Zhao, T.; Gu, J.; Pu, W.; Dong, Z.; Liu, R. Study on the synthesis and properties of an eco-friendly sugar-based anionic-nonionic surfactant. RSC Adv. 2016, 6, 70165–70173. [Google Scholar] [CrossRef]
- Han, N.S.; Heidelberg, T.; Salman, A.A. Spacer Effect on Triazole-Linked Sugar-Based Surfactants. J. Dispers. Sci. Technol. 2016, 2691, 105–109. [Google Scholar] [CrossRef]
- Krawczyk, J. Temperature impact on the water-air interfacial activity of n-octyl and n-dodecyl-β-D-glucopyranosides. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 61–67. [Google Scholar] [CrossRef]
- Baker, I.J.A.; Matthews, B.; Suares, H.; Krodkiewska, I.; Furlong, D.N.; Grieser, F.; Drummond, C.J. Sugar fatty acid ester surfactants: Structure and ultimate aerobic biodegradability. J. Surfactants Deterg. 2000, 3, 1–11. [Google Scholar] [CrossRef]
- Foley, P.; Pour, A.K.; Beach, E.S.; Zimmerman, J.B. Derivation and synthesis of renewable surfactants. Chem. Soc. Rev. 2012, 41, 1499–1518. [Google Scholar] [CrossRef]
- Neta, N.S.; Teixeira, J.A.; Rodrigues, L.R. Sugar Ester Surfactants: Enzymatic Synthesis and Applications in Food Industry. Crit. Rev. Food Sci. Nutr. 2015, 55, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Schramm, L.L.; Marangoni, G. Surfactants and Their Solutions: Basic Principles. In Surfactants: Fundamentals and Applications in the Petroleum Industry; Cambridge University Press: Cambridge, UK, 2000; pp. 3–50. [Google Scholar] [CrossRef]
- Weiss, J. Static and Dynamic Interfacial Tension Analysis. Curr. Protoc. Food Anal. Chem. 2003, 7, 1–16. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef]
- KRUSS Gmbh Germany. Critical Micelle Concentration (CMC) and Surfactant Concentration. (Kruss Scientific). Available online: http://kruss-scientific.com (accessed on 1 February 2021).
- Ahmad, A.; Kian, Y.S.; Lye, O.T.; Ahmad, S. Synergistic Effect Between Sodium Lauryl Sulphate and Sodium Lauryl Ether Sulphate With Alkyl. J. Oil Palm Res. 2007, 19, 332–337. [Google Scholar]
- Fluksman, A.; Benny, O. A robust method for critical micelle concentration determination using coumarin-6 as a fluorescent probe. Anal. Methods 2019, 11, 3810–3818. [Google Scholar] [CrossRef]
- Bazito, R.C.; El Seoud, O.A. Sugar-based anionic surfactants: Synthesis and micelleformation of sodium methyl2-acylamido-2-deoxy-6-O-sulfo-D-glucopyranosides. J. Surfactants Deterg. 2001, 4, 395–400. [Google Scholar] [CrossRef]
- Kovensky, J.; Grand, E. Recent Advances in the Synthesis of Sugar-based Surfactants. In Biomass Sugars for Non-Fuel Applications; Murzin, D., Simakova, O., Eds.; RSC Green Chemistry: London, UK, 2015; pp. 159–204. ISBN 978-1-78262-113-3. [Google Scholar]
- Basheva, E.S.; Kralchevsky, P.A.; Danov, K.D.; Ananthapadmanabhan, K.P.; Lips, A. The colloid structural forces as a tool for particle characterization and control of dispersion stability. Phys. Chem. Chem. Phys. 2007, 9, 5183–5198. [Google Scholar] [CrossRef] [PubMed]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Li, H.; Cai, K. Novel Sugar-Based Gemini Surfactants and Their Surface Properties. J. Surfactants Deterg. 2018, 21, 859–866. [Google Scholar] [CrossRef]
- Michaels, M.A. Quantitative Model for Prediction of Hydrodynamic Size of Nonionic Reverse Micelles. Master’s Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2007. [Google Scholar]
- Ge, X.; Zhang, S.; Chen, X.; Liu, X.; Qian, C. A designed bi-functional sugar-based surfactant: Micellar catalysis for C-X coupling reaction in water. Green Chem. 2019, 21, 2771–2776. [Google Scholar] [CrossRef]
- Bazito, R.C.; El Seoud, O.A. Sugar-based surfactants: Adsorption and micelle formation of sodium methyl 2-acylamido-2-deoxy-6-O-sulfo-D-glucopyranosides. Langmuir 2002, 18, 4362–4366. [Google Scholar] [CrossRef]
- Pan, A.; Mati, S.S.; Naskar, B.; Bhattacharya, S.C.; Moulik, S.P. Self-aggregation of MEGA-9 (N-Nonanoyl-N-methyl-d-glucamine) in aqueous medium: Physicochemistry of interfacial and solution behaviors with special reference to formation energetics and micelle microenvironment. J. Phys. Chem. B 2013, 117, 7578–7592. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.A.; Gratton, E.; Zanocco, A.L.; Lemp, E.; Gunther, G. Sucrose monoester micelles size determined by fluorescence correlation spectroscopy (FCS). PLoS ONE 2011, 6, e29278. [Google Scholar] [CrossRef] [PubMed]
- Porras, M.; Martínez, A.; Solans, C.; González, C.; Gutiérrez, J.M. Ceramic particles obtained using W/O nano-emulsions as reaction media. Colloids Surf. A Physicochem. Eng. Asp. 2005, 270–271, 189–194. [Google Scholar] [CrossRef]
- Lin, L.H.; Chu, H.C.; Chen, K.M.; Chen, S.C. Surface Properties of Glucose-Based Surfactants and Their Application in Textile Dyeing with Natural Dyes. J. Surfactants Deterg. 2019, 22, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Boullanger, P.; Chevalier, Y. Surface active properties and micellar aggregation of alkyl 2-amino-2-deoxy-β-D-glucopyranosides. Langmuir 1996, 12, 1771–1776. [Google Scholar] [CrossRef]
- Krawczyk, J. Aggregation properties of sucrose fatty acid esters and some other sugar-based surfactants at different temperatures. J. Mol. Liq. 2018, 271, 610–620. [Google Scholar] [CrossRef]
- Ducret, A.; Giroux, A.; Trani, M.; Lortie, R. Characterization of enzymatically prepared biosurfactants. JAOCS 1996, 73, 109–113. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J.; Yliruusi, J. The behavior of sorbitan surfactants at the water-oil interface: Straight-chained hydrocarbons from pentane to dodecane as an oil phase. J. Colloid Interface Sci. 2001, 240, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Smidrkal, J.; Cervenkova, R.; Filip, V. Two-stage synthesis of sorbitan esters, and physical properties of the products. Eur. J. Lipid Sci. Technol. 2004, 106, 851–855. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Adsorption and Aggregation Properties of Some Polysorbates at Different Temperatures. J. Solut. Chem. 2018, 47, 1824–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Zhang, J.; Yang, X.; Zhou, Y. Synthesis of Alkyl Monoglucoside Citric Monoester and Properties of Its Sodium Salt. J. Surfactants Deterg. 2016, 19, 885–891. [Google Scholar] [CrossRef]
- Szumała, P.; Mówińska, A. Perfectly Wetting Mixtures of Surfactants from Renewable Resources: The Interaction and Synergistic Effects on Adsorption and Micellization. J. Surfactants Deterg. 2016, 19, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Sułek, M.W.; Ogorzałek, M.; Wasilewski, T.; Klimaszewska, E. Alkyl polyglucosides as components of water based lubricants. J. Surfactants Deterg. 2013, 16, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Song, F.; Taxipalati, M.; Wei, W.; Feng, F.; Chen, C.S. Comparative study of surface-active properties and antimicrobial activities of disaccharide monoesters. PLoS ONE 2014, 9, e0114845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer, M.; Comelles, F.; Plou, F.J.; Cruces, M.A.; Fuentes, G.; Parra, J.L.; Ballesteros, A. Comparative surface activities of Di- and trisaccharide fatty acid esters. Langmuir 2002, 18, 667–673. [Google Scholar] [CrossRef]
- Krawczyk, J. Solid wettability modification via adsorption of antimicrobial sucrose fatty acid esters and some other sugar-based surfactants. Molecules 2018, 23, 1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salman, S.M. Sugar-Based Surfactants with Amide Linkage. Ph.D. Thesis, Thèse de L’Université de Malaisie, Kuala Lumpur, Malaysia, 2013. [Google Scholar]
- Burczyk, B.; Wilk, K.A.; Sokołowski, A.; Syper, L. Synthesis and surface properties of N-alkyl-N-methylgluconamides and N-alkyl-N-methyllactobionamides. J. Colloid Interface Sci. 2001, 240, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Dominguez, M.; Boutonnet, M.; Solans, C. A novel approach to metal and metal oxide nanoparticle synthesis: The oil-in-water microemulsion reaction method. J. Nanopart. Res. 2009, 11, 1823–1829. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Jin, Z.; Wang, W.; Wang, S. Synthesis of nanoiron by microemulsion with Span/Tween as mixed surfactants for reduction of nitrate in water. Front. Environ. Sci. Eng. China 2007, 1, 466–470. [Google Scholar] [CrossRef]
- Hada, R. A Novel Synthesis Process for Making Nickel Oxide Nanoparticles. Int. Res. J. Pure Appl. Chem. 2013, 3, 111–117. [Google Scholar] [CrossRef]
- Boutonnet, M.; Kizling, J.; Stenius, P.; Maire, G. The preparation of monodisperse colloidal metal particles from microemulsions. Colloids Surf. 1982, 5, 209–225. [Google Scholar] [CrossRef]
- Asta, M.; Kauzlarich, S.M.; Liu, K.; Navrotsky, A.; Osterloh, F.E. Inorganic Nanoparticles, Unique Properties and Novel Applications. Mater. Matters 2011, 2, 1–5. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/articles/material-matters/pdf/inorganic-nanoparticles.pdf (accessed on 3 March 2021).
- Lee, Y.; Lee, J.; Bae, C.J.; Park, J.G.; Noh, H.J.; Park, J.H.; Hyeon, T. Large-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditions. Adv. Funct. Mater. 2005, 15, 503–509. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef] [Green Version]
- Ganguli, A.K.; Ganguly, A.; Vaidya, S. Microemulsion-based synthesis of nanocrystalline materials. Chem. Soc. Rev. 2010, 39, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Jurek, A.; Kowalska, E.; Sobczak, J.W.; Lisowski, W.; Ohtani, B.; Zaleska, A. Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Appl. Catal. B Environ. 2011, 101, 504–514. [Google Scholar] [CrossRef]
- Langevin, D. Micelles and Microemulsions. Annu. Rev. Phys. Chem. 1992, 43, 341–369. [Google Scholar] [CrossRef]
- Yang, H. Synthesis and Characterization of Nanostructured Semiconductor Materials by Self-assembling Methods. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2008. [Google Scholar]
- Zhang, R.; Gao, L. Preparation of nanosized titania by hydrolysis of alkoxide titanium in micelles. Mater. Res. Bull. 2002, 37, 1659–1666. [Google Scholar] [CrossRef]
- Pineda-Reyes, A.M.; De La Olvera-Amador, M.L. Nanoparticles of zinc oxide obtained by water in oil microemulsion system. In Proceedings of the 13th International Conference on Electrical Engineering, Computing Science and Automatic Control, Mexico City, Mexico, 26–30 September 2016; pp. 5–6. [Google Scholar]
- Zhang, Z.; Xie, B.; Li, J.; Fang, B.; Lin, Y. CdS nanodots preparation and crystallization in a polymeric colloidal nanoreactor and their characterizations. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 203–211. [Google Scholar] [CrossRef]
- Khiew, P.S.; Huang, N.M.; Radiman, S.; Ahmad, M.S. Synthesis of NiS nanoparticles using a sugar-ester nonionic water-in-oil microemulsion. Mater. Lett. 2004, 58, 762–767. [Google Scholar] [CrossRef]
- Gilbert, R.G. Emulsion Polymerization: A Mechanistic Approach; Warson, H., Ed.; Academic Press: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Simon, M.O.; Li, C.J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Crass, H.; Ahrens, G. Use of Fatty Acid Glucamides as Emulsifiers in Emulsion Polymerization 2001. European Patent EP1072615A1, 31 January 2001. [Google Scholar]
- Chen, L.; Bao, Z.; Fu, Z.; Li, W. Characterization and particle size control of acrylic polymer latex prepared with green surfactants. Polym. Renew. Resour. 2015, 6, 65–74. [Google Scholar] [CrossRef]
- Bonham, J.A.; Faers, M.A.; Van Duijneveldt, J.S. Non-aqueous microgel particles: Synthesis, properties and applications. Soft Matter 2014, 10, 9384–9398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, A.; Scheider, D.; Grunder, S.; Krishnan, B. Inverse Emulsion Polymerization Process and Surfactant Composition Used Therefor. Chinese, World Intellectual Property Organization Patent WO2016187177A1, 24 November 2016. [Google Scholar]
- Wong, S.S.; Teng, T.T.; Ahmad, A.L.; Zuhairi, A.; Najafpour, G. Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J. Hazard. Mater. 2006, 135, 378–388. [Google Scholar] [CrossRef]
- Narayan, S.; Muldoon, J.; Finn, M.G.; Fokin, V.V.; Kolb, H.C.; Sharpless, K.B. “On water”: Unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 2005, 44, 3275–3279. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.J. Nanoreactors for polymerizations and organic reactions. Macromolecules 2010, 43, 1159–1168. [Google Scholar] [CrossRef]
- Gröger, H.; May, O.; Hüsken, H.; Georgeon, S.; Drauz, K.; Landfester, K. Enantioselective enzymatic reactions in miniemulsions as efficient “nanoreactors”. Angew. Chem. 2006, 45, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.E.; Peng, Y.; Chen, S. Janus Nanoparticle Emulsions as Chiral Nanoreactors for Enantiomerically Selective Ligand Exchange. Part. Part. Syst. Charact. 2019, 36, 1–5. [Google Scholar] [CrossRef]
- Qu, P.; Kuepfert, M.; Jockusch, S.; Weck, M. Compartmentalized Nanoreactors for One-Pot Redox-Driven Transformations. ACS Catal. 2019, 9, 2701–2706. [Google Scholar] [CrossRef]
- Biedermann, F.; Nau, W.M.; Schneider, H.J. The Hydrophobic Effect Revisited—Studies with Supramolecular Complexes Imply High-Energy Water as a Noncovalent Driving Force. Angew. Chem. 2014, 53, 11158–11171. [Google Scholar] [CrossRef]
- Engberts, J.B.F.N.; Blandamer, M.J. Understanding organic reactions in water: From hydrophobic encounters to surfactant aggregates. Chem. Commun. 2001, 1, 1701–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018, 118, 679–746. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Luque, R. An efficient renewable-derived surfactant for aqueous esterification reactions. RSC Adv. 2014, 4, 5152–5155. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef]
- Khan, Z.; Al-Thabaiti, S.A.; Malik, M.A. Biocompatible natural sugar-based surfactant assisted oxidation of citric acid by MnO4- in absence and presence of SDS. RSC Adv. 2016, 6, 45993–46001. [Google Scholar] [CrossRef]
- Kida, T.; Kajihara, K.; Isogawa, K.; Zhang, W.; Nakatsuji, Y.; Ikeda, I.; Akashi, M. Sugar-amide surfactant micelles as effective reaction fields for enantioselective hydrolysis of α-amino acid esters. Langmuir 2004, 20, 8504–8509. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chatterjee, A.; Roy, B.G.; Banerjee, M. Synthesis of novel D-glucose based anionic bolaamphiphiles and their catalytic application in 1,3-dipolar nitrone cycloaddition reactions. Catal. Commun. 2017, 94, 77–81. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Y.; Li, X.; Chen, X.; Lv, D.; Xie, C.; Yin, Q.; Zhang, X.; Hao, H. Confined crystallization of pigment red 146 in emulsion droplets and its mechanism. Nanomaterials 2019, 9, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgari, S.; Saberi, A.H.; McClements, D.J.; Lin, M. Microemulsions as nanoreactors for synthesis of biopolymer nanoparticles. Trends Food Sci. Technol. 2019, 86, 118–130. [Google Scholar] [CrossRef]
- Chin, S.F.; Azman, A.; Pang, S.C. Size Controlled Synthesis of Starch Nanoparticles by a Microemulsion Method. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.F.; Yazid, S.N.A.M.; Pang, S.C. Preparation and Characterization of Starch Nanoparticles for Controlled Release of Curcumin. Int. J. Polym. Sci. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Kobiasi, M.A.; Chua, B.Y.; Tonkin, D.; Jackson, D.C.; Mainwaring, D.E. Control of size dispersity of chitosan biopolymer microparticles and nanoparticles to influence vaccine trafficking and cell uptake. J. Biomed. Mater. Res. Part A 2012, 100, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Han, X.; He, L.; Deng, L.; Yu, K.; Jiang, H.; Wu, C.; Jia, Q.; Shan, S. Synthesis and characterization of magnetic dextran nanogel doped with iron oxide nanoparticles as magnetic resonance imaging probe. Int. J. Biol. Macromol. 2019, 128, 768–774. [Google Scholar] [CrossRef]
- Gao, Z.; Kwak, J.C.T.; Labonté, R.; Marangoni, D.G.; Wasylishen, R.E. Solubilization equilibria of alcohols and polymers in micellar solutions: NMR paramagnetic relaxation studies. Colloids Surf. 1990, 45, 269–281. [Google Scholar] [CrossRef]
- Jessop, P.G.; Ahmadpour, F.; Buczynski, M.A.; Burns, T.J.; Green, N.B.; Korwin, R.; Long, D.; Massad, S.K.; Manley, J.B.; Omidbakhsh, N.; et al. Opportunities for greener alternatives in chemical formulations. Green Chem. 2015, 17, 2664–2678. [Google Scholar] [CrossRef] [Green Version]
- Gaudin, T.; Rotureau, P.; Pezron, I.; Fayet, G. Investigating the impact of sugar-based surfactants structure on surface tension at critical micelle concentration with structure-property relationships. J. Colloid Interface Sci. 2018, 516, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Shamsi, S.A. Carbohydrate Based Polymeric Surfactants for Chiral Micellar Electrokinetic Chromatography (CMEKC) Coupled to Mass Spectrometry. Methods Mol. Biol. 2019, 1985, 417–444. [Google Scholar] [CrossRef]
- Vafakish, B.; Wilson, L.D. Surface Modified Chitosan: An Adsorption Study of a Biopolymer “Tweezer-Like” System with Fluorescein. Surfaces 2019, 2, 468–484. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Cazeneuve, C.; Baghdadli, N.; Ringeissen, S.; Leermakers, F.A.M.; Luengo, G.S. Surfactant-polymer interactions: Molecular architecture does matter. Soft Matter 2015, 11, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.A.; Loh, W. Liquid crystalline nanoparticles formed by oppositely charged surfactant-polyelectrolyte complexes. Curr. Opin. Colloid Interface Sci. 2017, 32, 11–22. [Google Scholar] [CrossRef]










| Glycan-Based Surfactant | Hydrodynamic Diameter (Rh; nm) | Reference |
|---|---|---|
| N-C14-lactosamine | 9 a | [53] |
| sodium methyl 2-dodecanoyl amido-2-deoxy-6-O-sulfo-D-glucopyranoside | 2.2 | [54] |
| sodium methyl 2-hexadecanoyl amido-2-deoxy-6-O-sulfo-D-glucopyranoside | 15.8 | [54] |
| N-nonanoyl-N-methyl-D-glucamine | 2.4 b | [55] |
| Stearic Acid Sucrose Monoester | 4.8 | [56] |
| Capric Acid Sucrose Monoester | 3.3 | [56] |
| Span-20, Tween-20 (60–40) with 5% water | 30 c | [57] |
| Glycan-Based Surfactant | CMC (mM) | Γmax (μmol/mL) | Amin (nm2) | γ (mN/m) | Reference |
|---|---|---|---|---|---|
| Sorbitan monooleate | 2.3 | 4 | 0.41 | 30 | [61,62,63] |
| Ethoxylated sorbitan monooleate | 0.20 | 1.4 | 0.12 | 37.5 | [62,63,64] |
| C10 Alkyl polyglycoside | 2.2 | 1.59 | - | 29 | [65,66,67] |
| C12–C14 Alkyl polyglycoside | 1.63 | 1.74 | 0.29 | 27 | [65,66,67] |
| Sucrose mono-decanoate | 0.60 | 17.6 | 0.09 | 26.9 | [68,69,70] |
| Sucrose monolaurate | 0.45 | 9.1 | 0.18 | 22 | [56,59,60,61] |
| Dodecanoyl methyl glucamide | 0.41 | - | - | 35.5 | [71,72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafakish, B.; Wilson, L.D. A Review on Recent Progress of Glycan-Based Surfactant Micelles as Nanoreactor Systems for Chemical Synthesis Applications. Polysaccharides 2021, 2, 168-186. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2010012
Vafakish B, Wilson LD. A Review on Recent Progress of Glycan-Based Surfactant Micelles as Nanoreactor Systems for Chemical Synthesis Applications. Polysaccharides. 2021; 2(1):168-186. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2010012
Chicago/Turabian StyleVafakish, Bahareh, and Lee D. Wilson. 2021. "A Review on Recent Progress of Glycan-Based Surfactant Micelles as Nanoreactor Systems for Chemical Synthesis Applications" Polysaccharides 2, no. 1: 168-186. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2010012