Effect of Cyanuric Acid as an Efficient Nucleating Agent on the Crystallization of Novel Biodegradable Branched Poly(Ethylene Succinate)
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Influence of CA on the Nonisothermal and Isothermal Melt Crystallization Behaviors of b-PES
3.2. Spherulitic Morphology and Crystal Structure Studies of Neat and Nucleated b-PES
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujimaki, T. Processability and Properties of Aliphatic Polyesters, ‘BIONOLLE’, Synthesized by Polycondensation Reaction. Polym. Degrad. Stab. 1998, 59, 209–214. [Google Scholar] [CrossRef]
- Ueda, A.; Chatani, Y.; Tadokoro, H. Structure Studies of Polyesters. IV. Molecular and Crystal Structure of Poly(ethylene succinate) and Poly(ethylene oxalate). Polym. J. 1971, 2, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Gan, Z.; Abe, H.; Doi, Y. Biodegradable Poly(ethylene succinate) (PES). 1. Crystal Growth Kinetics and Morphology. Biomacromolecules 2000, 1, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Abe, H.; Doi, Y. Biodegradable Poly(ethylene succinate) (PES). 2. Crystal Morphology of Melt-crystallized Ultrathin film and Its Change after Enzymatic Degradation. Biomacromolecules 2000, 1, 713–720. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Crystallization Behavior of Biodegradable Poly(ethylene succinate) from the Amorphous State. Polymer 2003, 44, 5429–5437. [Google Scholar] [CrossRef]
- Qiu, Z.; Fujinnami, S.; Komura, M.; Nakajima, K.; Ikehara, T.; Nishi, T. Nonisothermal Crystallization Kinetics of Poly(butylene succinate) and Poly(ethylene succinate). Polym. J. 2004, 36, 642–646. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, G.; Bikiaris, D.; Achilias, D. Effect of Molecular Weight on the Cold-crystallization of Biodegradable Poly(ethylene succinate). Thermochim. Acta 2007, 457, 41–54. [Google Scholar] [CrossRef]
- Qiu, Z.; Komura, M.; Ikehara, T.; Nishi, T. DSC and TMDSC study of Melting Behavior of Poly(butylene succinate) and Poly(ethylene succinate). Polymer 2003, 44, 7781–7785. [Google Scholar] [CrossRef]
- Iwata, T.; Doi, Y.; Isono, K.; Yoshida, Y. Morphology and Enzymatic Degradation of Solution-grown Single Crystals of Poly(ethylene succinate). Macromolecules 2001, 34, 7343–7348. [Google Scholar] [CrossRef]
- Tezuka, Y.; Ishii, N.; Kasuya, K.; Mitomo, H. Degradation of Poly(ethylene succinate) by Mesophilic Bacteria. Polym. Degrad. Stab. 2004, 84, 115–121. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Bikiaris, D. Synthesis and Properties of Novel Biodegradable/Biocompatible Poly[propyleneco-(ethylene succinate)] Random Copolyesters. Macromol. Chem. Phys. 2009, 210, 1408–1421. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Z. Crystallization and Melting Behavior of Biodegradable Poly(ethylene succinate-co-6mol% butylene succinate). J. Appl. Polym. Sci. 2011, 122, 105–111. [Google Scholar] [CrossRef]
- Li, X.; Qiu, Z. Crystallization Kinetics, Morphology, and Mechanical Properties of Novel Poly(ethylene succinate-co-octamethylene succinate). Polym. Test. 2015, 48, 125–132. [Google Scholar] [CrossRef]
- Li, X.; Qiu, Z. Synthesis and Properties of Novel Poly(ethylene succinate-co-decamethylene succinate) Copolymers. RSC Adv. 2015, 5, 103713–103721. [Google Scholar] [CrossRef]
- Wu, H.; Qiu, Z. Synthesis, Crystallization Kinetics and Morphology of Novel Poly(ethylene succinate-co-ethylene adipate) Copolymers. CrystEngComm 2012, 14, 3586–3595. [Google Scholar] [CrossRef]
- Qiu, S.; Su, Z.; Qiu, Z. Crystallization Kinetics, Morphology, and Mechanical Properties of Novel Biodegradable Poly(ethylene succinate-co-ethylene suberate) Copolyesters. Ind. Eng. Chem. Res. 2016, 55, 10286–10293. [Google Scholar] [CrossRef]
- Qiu, S.; Su, Z.; Qiu, Z. Isothermal and Nonisothermal Crystallization Kinetics of Novel Biobased Poly(ethylene succinate-co-ethylene sebacate) Copolymers from the Amorphous State. J. Therm. Ana. Calorim. 2017, 129, 801–808. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, K.; Su, Z.; Qiu, Z. Thermal Behavior, Mechanical and Rheological Properties, and Hydrolytic Degradation of Novel Branched Biodegradable Poly(ethylene succinate) Copolymers. Polym. Test. 2018, 66, 64–69. [Google Scholar] [CrossRef]
- Kim, M.; Kim, K.; Jin, H.; Park, J.; Yoon, J. Biodegradability of Ethyl and n-octyl Branched Poly(ethylene adipate) and Poly(butylene succinate). Eur. Polym. J. 2001, 37, 1843–1847. [Google Scholar] [CrossRef]
- Kim, E.; Bae, J.; Im, S.; Kim, B.; Han, Y. Preparation and Properties of Branched Polybutylenesuccinate. J. Appl. Polym. Sci. 2001, 80, 1388–1394. [Google Scholar] [CrossRef]
- Jin, H.; Kim, D.; Kim, M.; Lee, I.; Lee, H.; Yoon, J. Synthesis and Properties of Poly(butylene succinate) with n-hexenyl Side Branches. J. Appl. Polym. Sci. 2001, 81, 2219–2226. [Google Scholar] [CrossRef]
- Chae, H.; Park, S.; Kim, B.; Kim, D. Effect of Methyl Substitution of the Ethylene Unit on the Physical Properties of Poly(butylene succinate). J. Polym. Sci. Polym. Phys. 2004, 42, 1759–1766. [Google Scholar] [CrossRef]
- Wang, G.; Gao, B.; Ye, H.; Xu, J.; Guo, B. Synthesis and Characterizations of Branched Poly(butylene succinate) Copolymers with 1,2-octanediol Segments. J. Appl. Polym. Sci. 2010, 117, 2538–2544. [Google Scholar] [CrossRef]
- Pan, P.; Shan, G.; Bao, Y.; Weng, Z. Crystallization Kinetics of Bacterial Poly(3-hydroxylbutyrate) Copolyesters with Cyanuric Acid as A Nucleating Agent. J. Appl. Polym. Sci. 2013, 129, 1374–1382. [Google Scholar] [CrossRef]
- Weng, M.; Qiu, Z. Effect of Cyanuric Acid on The Crystallization Kinetics and Morphology of Biodegradable Poly(L-lactide) as an Efficient Nucleating Agent. Thermochimi. Acta 2014, 577, 41–45. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Qin, S.; Liu, J.; Bi, C.; Liang, R.; Dong, T.; Feng, X. Effects of Cyanuric Acid on Crystallization Behavior, Polymorphism, and Phase Transition of Poly(butylene adipate). Ind. Eng. Chem. Res. 2015, 54, 8048. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, Z. Enhanced Crystallization Rate of Biodegradable Poly(ε-caprolactone) by Cyanuric Acid as an Efficient Nucleating Agent. Chin. J. Polym. Sci. 2017, 35, 1517–1523. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Bikiaris, D. Crystallization and Melting Behavior of Three Biodegradable Poly(alkylene succinates). A Comparative Study. Polymer 2005, 46, 12081–12092. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Verschoor, G.; Keulen, E. Electron Density Distribution in Cyanuric Acid. I. An X-ray Diffraction Study at Low Temperature. Acta Cryst. 1971, B27, 134–145. [Google Scholar] [CrossRef]

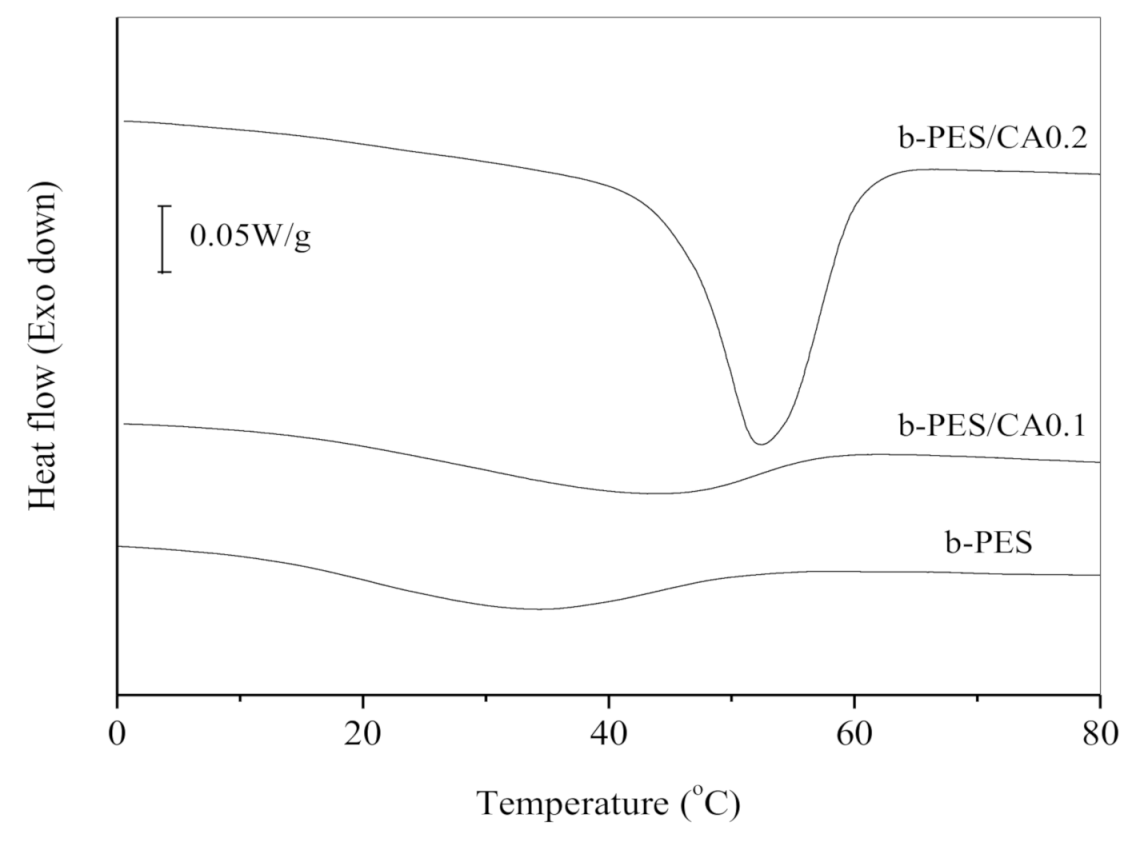


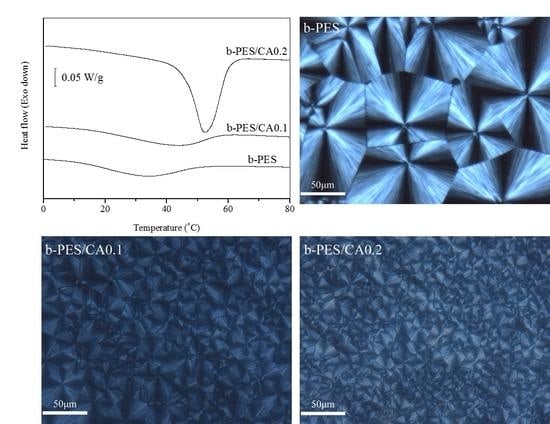
| Samples | b-PES | b-PES/CA0.1 | b-PES/CA0.2 |
|---|---|---|---|
| Tp (°C) | 33.4 | 43.1 | 52.3 |
| ΔHc (J/g) | 10.7 | 11.5 | 30.4 |
| Xc (%) | 6.0 | 6.4 | 16.9 |
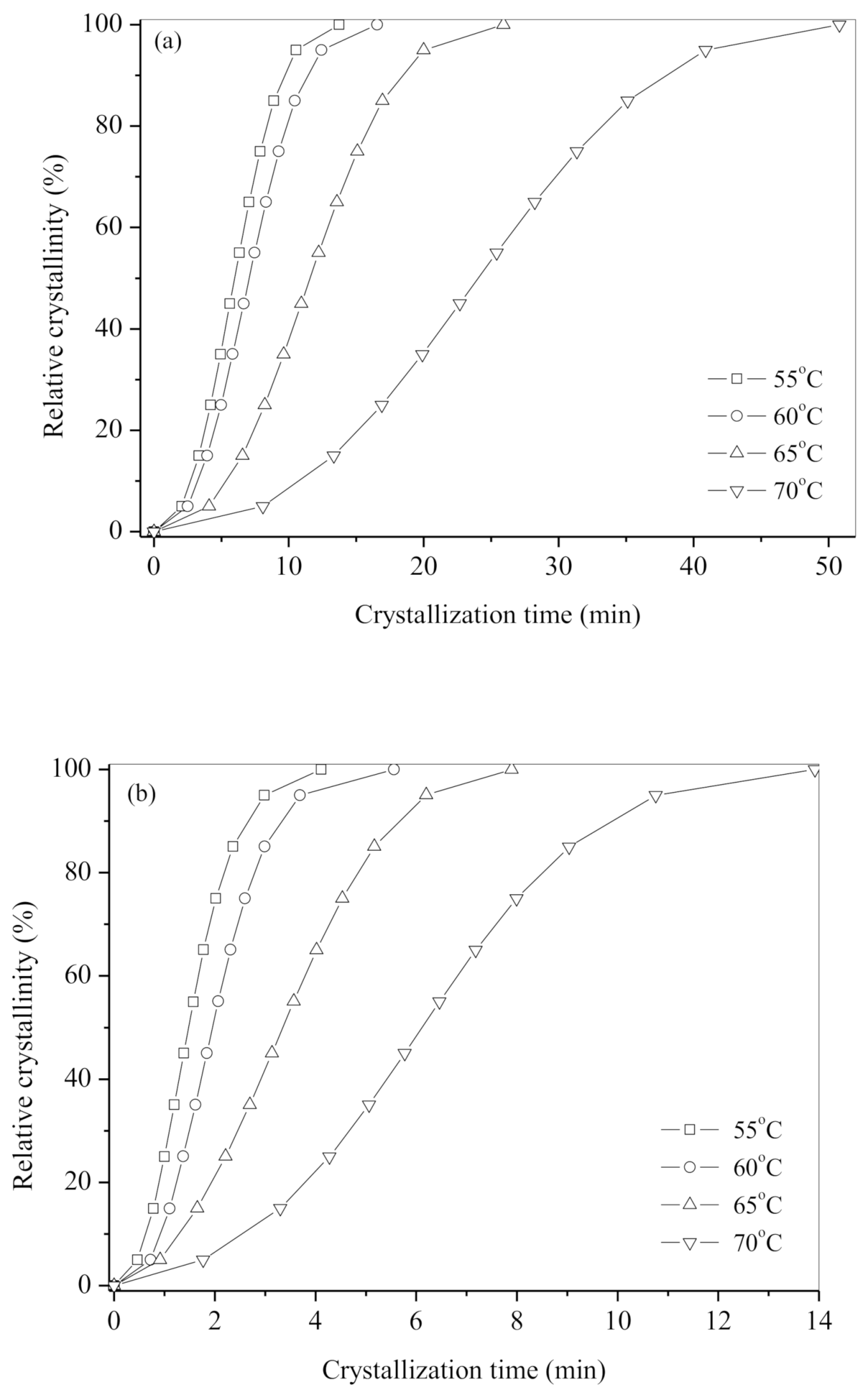
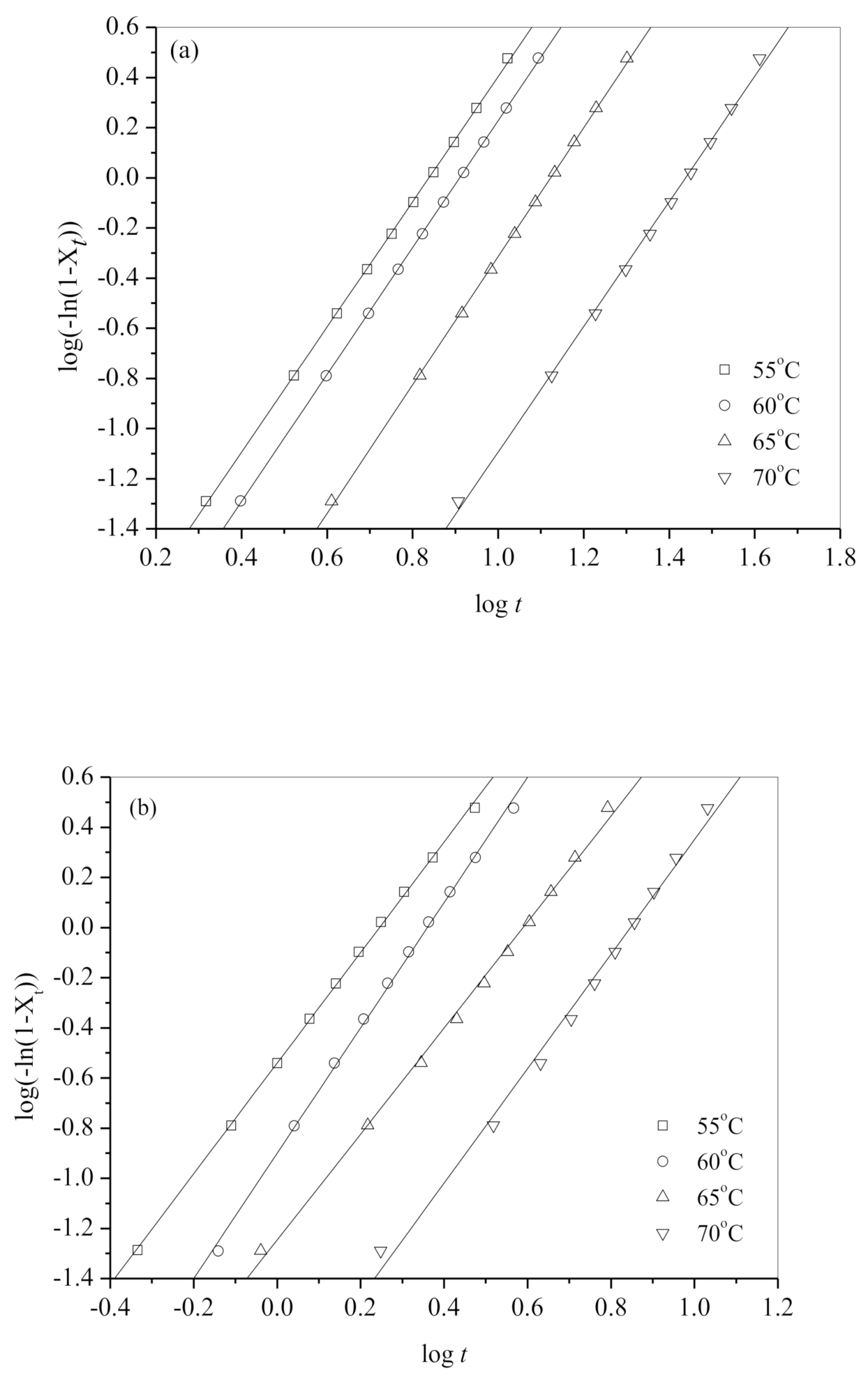
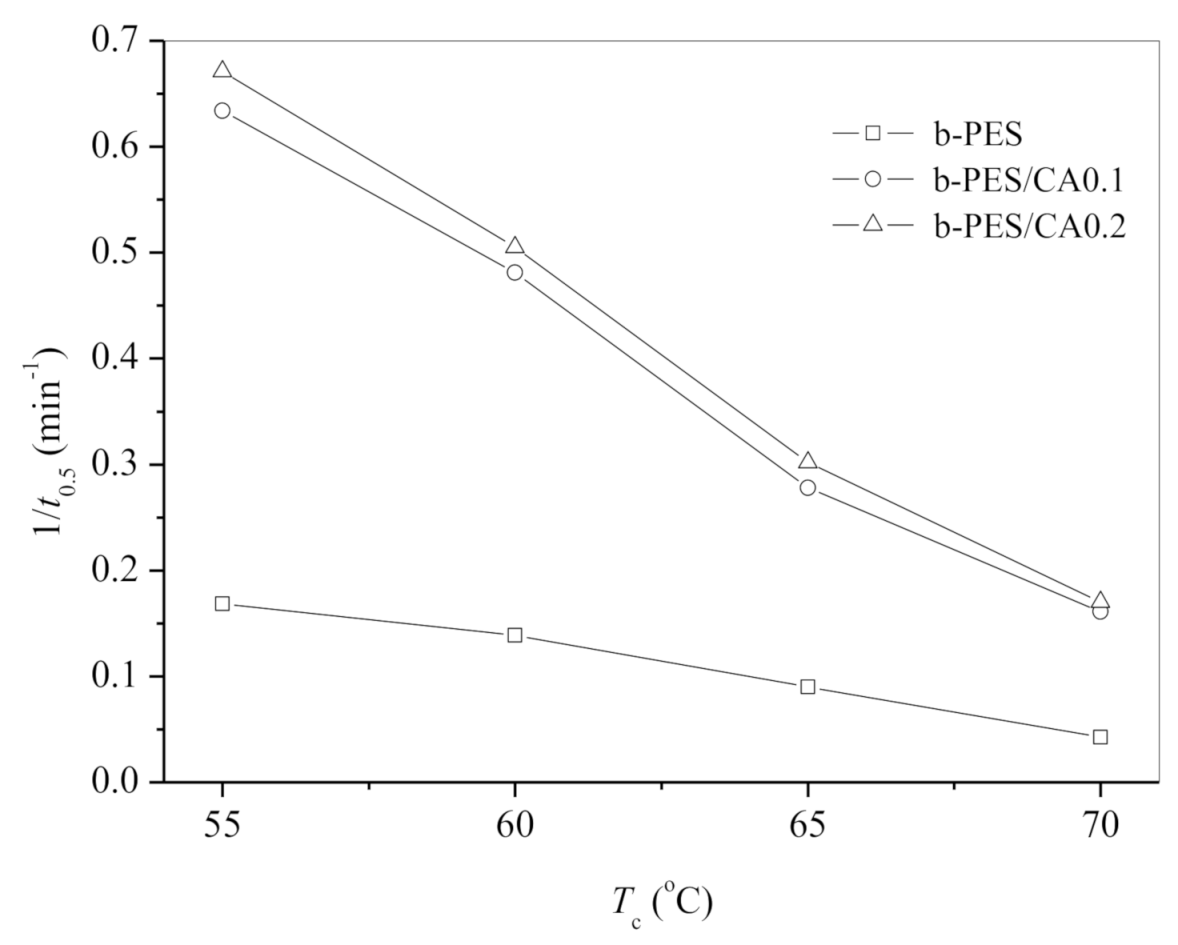
| Tc (°C) | n | k (min−n) | t0.5 (min) | |
|---|---|---|---|---|
| Neat b-PES | 55 | 2.5 | 8.07 × 10−3 | 5.94 |
| 60 | 2.5 | 4.97 × 10−3 | 7.21 | |
| 65 | 2.6 | 1.33 × 10−3 | 11.08 | |
| 70 | 2.5 | 2.57 × 10−4 | 23.58 | |
| b-PES/CA0.1 | 55 | 1.9 | 2.92 × 10−1 | 1.58 |
| 60 | 2.0 | 1.61 × 10−1 | 2.08 | |
| 65 | 2.1 | 4.72 × 10−2 | 3.59 | |
| 70 | 1.9 | 2.15 × 10−2 | 6.22 | |
| b-PES/CA0.2 | 55 | 2.2 | 2.88 × 10−1 | 1.49 |
| 60 | 2.5 | 1.26 × 10−1 | 1.98 | |
| 65 | 2.1 | 5.62 × 10−2 | 3.31 | |
| 70 | 2.3 | 1.17 × 10−2 | 5.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Qiu, Z. Effect of Cyanuric Acid as an Efficient Nucleating Agent on the Crystallization of Novel Biodegradable Branched Poly(Ethylene Succinate). Macromol 2021, 1, 112-120. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1020009
Zhang K, Qiu Z. Effect of Cyanuric Acid as an Efficient Nucleating Agent on the Crystallization of Novel Biodegradable Branched Poly(Ethylene Succinate). Macromol. 2021; 1(2):112-120. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1020009
Chicago/Turabian StyleZhang, Kangjing, and Zhaobin Qiu. 2021. "Effect of Cyanuric Acid as an Efficient Nucleating Agent on the Crystallization of Novel Biodegradable Branched Poly(Ethylene Succinate)" Macromol 1, no. 2: 112-120. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1020009