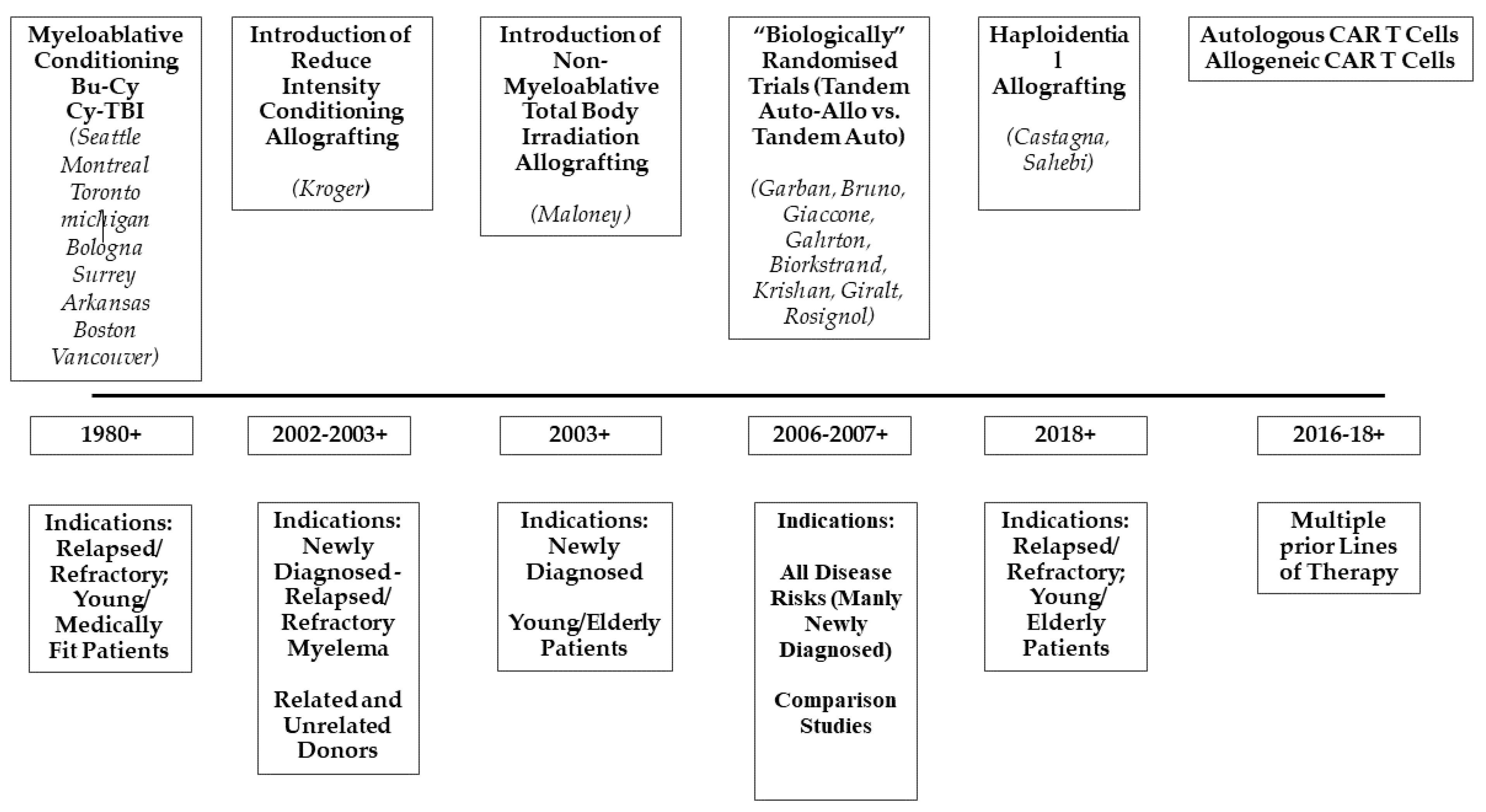
Decades of Progress in Allogeneic Stem Cell Transplantation for Multiple Myeloma
Abstract
:1. Introduction
2. The First Reports: Myeloablative Conditionings and Their Toxicity
3. The Concept of Tandem Autologous-Allogeneic Transplantation and Reduced-Intensity Conditioning Regimens
4. Haplo-Identical Transplantation
5. Allografting in Refractory/Relapsed Myeloma and High-Risk Disease
6. Allogeneic Transplantation and Novel Agents: An Immunological Synergy
7. Minimal Residual Disease (MRD) and Graft-vs.-Myeloma
8. Graft-vs.-Myeloma Effects: The New Frontiers
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Main, J.M.; Prehn, R.T. Success skin homografts after the administration of high dosage X radiation and homologous bone marrow. J. Natl. Cancer Inst. 1955, 15, 1023–1029. [Google Scholar]
- Buckner, C.D.; Clift, R.A.; Fefer, A.; Neiman, P.; Storb, R.; Thomas, E.D. Human marrow transplantation--current status. Prog. Hematol. 1973, 8, 299–324. [Google Scholar]
- Thomas, E.; Storb, R.; Clift, R.A.; Fefer, A.; Johnson, F.L.; Neiman, P.E.; Lerner, K.G.; Glucksberg, H.; Buckner, C.D. Bone-marrow transplantation (first of two parts). N. Engl. J. Med. 1975, 292, 832–843. [Google Scholar] [CrossRef]
- Thomas, E.D. The Nobel Prize in Physiology or Medicine 1990. In Les Prix Nobel, the Nobel Prizes 1990; Frängsmyr, T., Ed.; Nordstedts Tryckeri AB: Stockholm, Sweden, 1991; pp. 219–221. [Google Scholar]
- Gahrton, G.; Ringden, O.; Lönnqvist, B.; Lindquist, R.; Ljungman, P. Bone marrow transplantation in three patients with multiple myeloma. Acta Med. Scand. 1986, 219, 523–527. [Google Scholar] [CrossRef]
- Tura, S. Bone marrow transplantation in multiple myeloma: Current status and future perspectives. Bone Marrow Transpl. 1986, 1, 17–20. [Google Scholar]
- Gahrton, G.; Tura, S.; Ljungman, P.; Belanger, C.; Brandt, L.; Cavo, M.; Facon, T.; Granena, A.; Gore, M.; Gratwohl, A.; et al. Allogeneic bone marrow transplantation in multiple myeloma. N. Engl. J. Med. 1991, 325, 1267–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensinger, W.I.; Buckner, C.D.; Anasetti, C.; Clift, R.; Storb, R.; Barnett, T.; Chaunett, T.; Shulman, H.; Appelbaum, F.R. Allogeneic marrow transplantation for multiple myeloma: An analysis of risk factors on outcome. Blood 1996, 88, 2787–2793. [Google Scholar] [CrossRef] [Green Version]
- Gahrton, G.; Tura, S.; Ljungman, P.; Biadé, J.; Brandt, L.; Cavo, M.; Façon, T.; Gratwohl, A.; Hagenbeek, A.; Jacobs, P.; et al. Prognostic factors in allogeneic bone marrow transplantation for multiple myeloma. J. Clin. Oncol. 1995, 13, 1312–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlogie, B.; Kyle, R.A.; Anderson, K.C.; Greipp, P.R.; Lazarus, H.M.; Hurd, D.D.; McCoy, J.; Moore Jr, D.F.; Dakhil, S.R.; Lanier, K.S.; et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: Final results of phase III US Intergroup Trial S9321. J. Clin. Oncol. 2006, 24, 929–936. [Google Scholar] [CrossRef]
- Bensinger, W.I.; Maloney, D.; Storb, R. Allogeneic hematopoietic cell transplantation for multiple myeloma. Semin. Hemalol. 2001, 38, 243–249. [Google Scholar]
- Reece, D.E.; Shepherd, J.D.; Klingemann, H.G.; Sutherland, H.J.; Nantel, S.H.; Barnett, M.J.; Spinelli, J.J.; Phillips, G.L. Treatment of myeloma using intensive therapy and allogeneic bone marrow transplantation. Bone Marrow Transpl. 1995, 15, 117–123. [Google Scholar]
- Alyea, E.; Weller, E.; Schlossman, R.; Canning, C.; Webb, I.; Doss, D.; Mauch, P.; Marcus, K.; Fisher, D.; Freeman, A.; et al. T-cell-depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: Induction of graft-versus-myeloma effect. Blood 2001, 98, 934–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, S.; Powles, R.L.; Treleaven, J.G.; Singhal, S.; Saso, R.; Horton, C.; Killick, S.; Tait, D.; Ramiah, V.; Mehta, J. Impact of previous high-dose therapy on outcome after allografting for multiple myeloma. Bone Marrow Transpl. 1999, 23, 675–680. [Google Scholar] [CrossRef]
- Le Blanc, R.; Montminy-Métivier, S.; Bélanger, R.; Busque, L.; Fish, D.; Roy, D.-C.; Kassis, J.; Boileau, J.; Lavallée, R.; Bélanfer, F.; et al. Allogeneic transplantation for multiple myeloma: Further evidence for a GVHD-associated graft-versus-myeloma effect. Bone Marrow Transpl. 2001, 28, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Couban, S.; Stewart, A.K.; Loach, D.; Panzarella, T.; Meharchand, J. Autologous and allogeneic transplantation for multiple myeloma at a single centre. Bone Marrow Transpl. 1997, 19, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Varterasian, M.; Janakiraman, N.; Karanes, C.; Aberlla, E.; Uberti, J.; Dragovic, J.; Raman, S.B.K.; Al-Katib, A.; Du, W.; Silver, S.M.; et al. Transplantation in patients with multiple myeloma: A multicenter comparative analysis of peripheral blood stem cell and allogeneic transplant. Am. J. Clin. Oncol. 1997, 20, 462–466. [Google Scholar] [CrossRef]
- Gahrton, G.; Svensson, H.; Cavo, M.; Apperley, J.; Bacigalupo, A.; Björkstrand, B.; Blade, J.; Cornelissen, J.; De Laurenzi, A.; Façon, T.; et al. Progress in allogeneic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: A comparison between transplants performed 1983–93 and 1994–98 at European Group for Blood and Marrow Transplantation centres. Br. J. Haematol. 2001, 113, 209–216. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, P.A.; Niederwieser, D.; Shizuru, J.A.; Sandmaier, B.M.; Molina, A.J.; Maloney, D.G.; Chauncey, T.R.; Gooley, T.A.; Hegenbart, U.; Nash, R.A.; et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: Replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001, 97, 3390–3400. [Google Scholar] [CrossRef] [Green Version]
- Maloney, D.G.; Molina, A.J.; Sahebi, F.; Stockerl-Goldstein, K.E.; Sandmaier, B.M.; Bensinger, W.; Storer, B.; Hegenbart, U.; Somlo, G.; Chauncey, T.; et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood 2003, 102, 3447–3454. [Google Scholar] [CrossRef] [Green Version]
- Kröger, N.; Schwerdtfeger, R.; Kiehl, M.; Sayer, H.G.; Renges, H.; Zabelina, T.; Fehse, B.; Tögel, F.; Wittkowsky, G.; Kuse, R.; et al. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood 2002, 100, 755–760. [Google Scholar] [CrossRef]
- Kröger, N.; Sayer, H.G.; Schwerdtfeger, R.; Kiehl, M.; Nagler, A.; Renges, H.; Zabelina, T.; Fehse, B.; Ayuk, F.; Wittkowsky, G.; et al. Unrelated stem cell transplantation in multiple myeloma after a reduced- intensity conditioning with pretransplantation antithymocyte globulin is highly effective with low transplantation-related mortality. Blood 2002, 100, 3919–3924. [Google Scholar] [CrossRef] [Green Version]
- Garban, F.; Attal, M.; Michallet, M.; Hulin, C.; Bourhis, J.H.; Yakoub-Agha, I.; Lamy, T.; Marit, G.; Maloisel, F.; Berthou, C.; et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood 2006, 107, 3474–3480. [Google Scholar] [CrossRef] [Green Version]
- Moreau, P.; Garban, F.; Attal, M.; Michallet, M.; Marit, G.; Hulin, C.; Benboubker, L.; Doyen, C.; Mohty, M.; Yakoub-Agha, I.; et al. Long-term follow-up results of IFM99-03 and IFM99-04 trials comparing nonmyeloablative allotransplantation with autologous transplantation in high-risk de novo multiple myeloma. Blood 2008, 112, 3914–3915. [Google Scholar] [CrossRef]
- Bruno, B.; Rotta, M.; Patriarca, F.; Mordini, N.; Allione, B.; Carnevale-Schianca, F.; Giaccone, L.; Sorasio, R.; Omedé, P.; Baldi, I.; et al. A Comparison of allografting with autografting for newly diagnosed myeloma. N. Engl. J. Med. 2007, 356, 1110–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaccone, L.; Storer, B.; Patriarca, F.; Rotta, M.; Sorasio, R.; Allione, B.; Carnevale-Schianca, F.; Festuccia, M.; Brunello, L.; Omedè, P.; et al. Long-term follow-up of a comparison of nonmyeloablative allografting with autografting for newly diagnosed myeloma. Blood 2011, 117, 6721–6727. [Google Scholar] [CrossRef] [Green Version]
- Rosiñol, L.; Perez-Simón, J.A.; Sureda, A.; Rubia, J.D.L.; De Arriba, F.; Lahuerta, J.J.; González, J.D.; Diaz-Mediavilla, J.; Hernández, B.; García-Frade, J.; et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood 2008, 112, 3591–3593. [Google Scholar] [CrossRef] [Green Version]
- Lokhorst, H.M.; Van Der Holt, B.; Cornelissen, J.J.; Kersten, M.J.; Van Oers, M.; Raymakers, R.; Minnema, M.C.; Zweegman, S.; Janssen, J.J.; Zijlmans, M.; et al. Donor versus no-donor comparison of newly diagnosed myeloma patients included in the HOVON-50 multiple myeloma study. Blood 2012, 119, 6219–6225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokhorst, H.M.; Van Der Holt, B.; Cornelissen, J.J.; Kersten, M.J.; Van Oers, M.; Raymakers, R.; Minnema, M.C.; Zweegman, S.; Bos, G.; Schaap, N.; et al. Reduced relapse rate in upfront tandem autologous/reduced-intensity allogeneic transplantation in multiple myeloma only results in borderline non-significant prolongation of progression-free but not overall survival. Haematologica 2015, 100, e508–e510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, A.; Pasquini, M.C.; Logan, B.; Stadtmauer, E.A.; Vesole, D.H.; Alyea, E.; Antin, J.H.; Comenzo, R.; Goodman, S.; Hari, P.; et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): A phase 3 biological assignment trial. Lancet Oncol. 2011, 12, 1195–1203. [Google Scholar] [CrossRef] [Green Version]
- Giralt, S.A.; Costa, L.J.; Maloney, D.; Krishnan, A.; Fei, M.; Antin, J.H.; Brunstein, C.; Geller, N.; Goodman, S.; Hari, P.; et al. Tandem autologous-autologous versus autologous-allogeneic hematopoietic stem cell transplant for patients with multiple myeloma: Long-term follow-up results from the Blood and Marrow Transplant Clinical Trials Network 0102 Trial. Biol. Blood Marrow Transpl. 2020, 26, 798–804. [Google Scholar] [CrossRef]
- Björkstrand, B.; Iacobelli, S.; Hegenbart, U.; Gruber, A.; Greinix, H.; Volin, L.; Narni, F.; Musto, P.; Beksac, M.; Bosi, A.; et al. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: Long-term follow-up. J. Clin. Oncol. 2011, 29, 3016–3022. [Google Scholar] [CrossRef]
- Gahrton, G.; Iacobelli, S.; Björkstrand, B.; Hegenbart, U.; Gruber, A.; Greinix, H.; Volin, L.; Narni, F.; Carella, A.M.; Beksac, M.; et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: Long-term results of the EBMT-NMAM2000 study. Blood 2013, 121, 5055–5063. [Google Scholar] [CrossRef] [Green Version]
- Armenson, K.E.; Hill, E.G.; Costa, L.J. Tandem autologous vs autologous plus reduced intensity allogeneic transplantation in the upfront management of multiple myeloma: Meta-analysis of trials with biological assignment. Bone Marrow Transpl. 2013, 48, 562–567. [Google Scholar] [CrossRef]
- Sahebi, F.; Garderet, L.; Kanate, A.S.; Eikema, D.J.; Knelange, N.S.; Alvelo, O.F.D.; Koc, Y.; Blaise, D.; Bashir, Q.; Moraleda, J.M.; et al. Outcomes of Haploidentical Transplantation in Patients with elapsed Multiple Myeloma: An EBMT/CIBMTR Report. Biol. Blood Marrow Transpl. 2019, 25, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, S.R.; Chakrabarti, S. Natural killer cell-based immunotherapy with CTLA4Ig-primed donor lymphocytes following haploidentical transplantation. Immunotherapy 2019, 11, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Sobh, M.; Michallet, M.; Gahrton, G.; Iacobelli, S.; Van Biezen, A.; Schönland, S.O.; Petersen, E.; Schaap, N.; Bonifazi, F.; Volin, L.; et al. Allogeneic hematopoietic cell transplantation for multiple myeloma in Europe: Trends and outcomes over 25 years. A study by the EBMT Chronic Malignancies Working Party. Leukemia 2016, 30, 2047–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.J.M.; Iacobelli, S.; Pasquini, M.C.; Modi, R.; Giaccone, L.; Blade, J.; Schonland, S.; Evangelista, A.; Perez-Simon, J.A.; Hari, P.; et al. Long-term survival of 1338 MM patients treated with tandem autologous vs. autologous-allogeneic transplantation. Bone Marrow Transpl. 2020, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Castagna, L.; Mussetti, A.; DeVillier, R.; Dominietto, A.; Marcatti, M.; Milone, G.; Maura, F.; De Philippis, C.; Bruno, B.; Furst, S.; et al. Haploidentical allogeneic hematopoietic cell transplantation for multiple myeloma using post-transplantation cyclophosphamide graft-versus-host disease prophylaxis. Biol. Blood Marrow Transpl. 2017, 23, 1549–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efebera, Y.A.; Qureshi, S.R.; Cole, S.M.; Saliba, R.; Pelosini, M.; Patel, R.M.; Koca, E.; Mendoza, F.L.; Wang, M.; Shah, J.; et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed multiple myeloma. Biol. Blood Marrow Transpl. 2010, 16, 1122–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlin, L.; Arnulf, B.; Chevret, S.; Ades, L.; Robin, M.; De Latour, R.P.; Malphettes, M.; Kabbara, N.; Asli, B.; Rocha, V.; et al. Tandem autologous non-myeloablative allogeneic transplantation in patients with multiple myeloma relapsing after a first high dose therapy. Bone Marrow Transpl. 2010, 46, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Shimoni, A.; Hardan, I.; Ayuk, F.; Schilling, G.; Atanackovic, D.; Zeller, W.; Yerushalmi, R.; Zander, A.R.; Kröger, N.; Nagler, A. Allogenic hematopoietic stem-cell transplantation with reduced-intensity conditioning in patients with refractory and recurrent multiple myeloma. Cancer 2010, 116, 3621–3630. [Google Scholar] [CrossRef]
- Patriarca, F.; Einsele, H.; Spina, F.; Bruno, B.; Isola, M.; Nozzoli, C.; Nozza, A.; Sperotto, A.; Morabito, F.; Stuhler, G.; et al. Allogeneic stem cell transplantation in multiple myeloma relapsed after autograft: A multicenter retrospective study based on donor availability. Biol Blood Marrow Transpl. 2012, 18, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Patriarca, F.; Bruno, B.; Einsele, H.; Spina, F.; Giaccone, L.; Montefusco, V.; Isola, M.; Nozzoli, C.; Nozza, A.; Morabito, F.; et al. Long-term follow-up of a Donor versus No-Donor Comparison in patients with multiple myeloma in first relapse after failing autologous transplantation. Biol. Blood Marrow Transpl. 2018, 24, 406–409. [Google Scholar] [CrossRef] [Green Version]
- Giralt, S.A.; Garderet, L.; Durie, B.; Cook, G.; Gahrton, G.; Bruno, B.; Hari, P.; Lokhorst, H.; McCarthy, P.; Krishnan, A.; et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on salvage hematopoietic cell transplantation in patients with relapsed multiple myeloma. Biol. Blood Marrow Transpl. 2015, 21, 2039–2051. [Google Scholar]
- Knop, S.; Engelhardt, M.; Liebisch, P.; Meisner, C.; Holler, E.; Metzner, B.; Peest, D.; Kaufmann, M.; Bunjes, D.; Straka, C.; et al. Allogeneic transplantation in multiple myeloma: Long-term follow-up and cytogenetic subgroup analysis. SSRN Electron. J. 2019, 33, 2710–2719. [Google Scholar]
- Kröger, N.; Badbaran, A.; Zabelina, T.; Ayuk, F.; Wolschke, C.; Alchalby, H.; Klyuchnikov, E.; Atanackovic, D.; Schilling, G.; Hansen, T.; et al. Impact of high-risk cytogenetics and achievement of molecular remission on long-term freedom from disease after autologous-allogeneic tandem transplantation in patients with multiple myeloma. Biol. Blood Marrow Transpl. 2013, 19, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Htut, M.; D’Souza, A.; Krishnan, A.; Bruno, B.; Zhang, M.-J.; Fei, M.; González-Díaz, M.; Copelan, E.; Ganguly, S.; Hamadani, M.; et al. Autologous/Allogeneic hematopoietic cell transplantation versus tandem autologous transplantation for multiple myeloma: Comparison of long-term postrelapse survival. Biol. Blood Marrow Transpl. 2018, 24, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Kneppers, E.; Van Der Holt, B.; Kersten, M.J.; Zweegman, S.; Meijer, E.; Huls, G.; Cornelissen, J.J.; Janssen, J.J.; Huisman, C.; Cornelisse, P.B.; et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: Results of the HOVON 76 Trial. Blood 2011, 118, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Wolschke, C.; Stübig, T.; Hegenbart, U.; Schönland, S.O.; Heinzelmann, M.; Hildebrandt, Y.; Ayuk, F.; Atanackovic, D.; Dreger, P.; Zander, A.; et al. Postallograft lenalidomide induces strong NK cell–mediated antimyeloma activity and risk for T cell-mediated GvHD: Results from a phase I/II dose-finding study. Exp. Hematol. 2013, 41, 134–142.e3. [Google Scholar] [CrossRef]
- Libura, J.; Hoffmann, T.; Passweg, J.R.; Gregor, M.; Favre, G.; Tichelli, A.; Gratwohl, A. Graft-versusmyeloma after withdrawal of immunosuppression following allogeneic peripheral stem cell transplantation. Bone Marrow Transpl. 1999, 24, 925–927. [Google Scholar] [CrossRef] [Green Version]
- Verdonck, L.F.; Lokhorst, H.M.; Dekker, A.W.; Nieuwenhuis, H.K.; Petersen, E.J. Graft-versus-myeloma effect in two cases. Lancet 1996, 347, 800–801. [Google Scholar] [CrossRef]
- Lokhorst, H.M.; Schattenberg, A.; Cornelissen, J.J.; Thomas, L.L.M.; Verdonck, L.F. Donor leukocyte infusions are effective in relapsed multiple myeloma after allogeneic bone marrow transplantation. Blood 1997, 90, 4206–4211. [Google Scholar] [CrossRef]
- Lokhorst, H.M.; Schattenberg, A.; Cornelissen, J.J.; van Oers, M.H.J.; Fibbe, W.; Van De Donk, N.W.C.J.; Verdonck, L.F. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: Predictive factors for response and long-term outcome. J. Clin. Oncol. 2000, 18, 3031–3037. [Google Scholar] [CrossRef]
- Lokhorst, H.; Wu, K.; Verdonck, L.F.; Laterveer, L.L.; Van De Donk, N.W.C.J.; Van Oers, M.H.J.; Cornelissen, J.J.; Schattenberg, A.V. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood 2004, 103, 4362–4364. [Google Scholar] [CrossRef] [Green Version]
- Van de Donk, N.W.; Kröger, N.; Hegenbart, U.; Corradini, P.; Miguel, J.F.S.; Goldschmidt, H.; Perez-Simon, J.A.; Zijlmans, M.; Raymakers, R.A.; Montefusco, V.; et al. Prognostic factors for donor lymphocyte infusions following nonmyeloablative allogeneic stem cell transplantation in multiple myeloma. Bone Marrow Transpl. 2006, 37, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Rawstron, A.C.; Davies, F.E.; DasGupta, R.; Ashcroft, A.J.; Patmore, R.; Drayson, M.T.; Owen, R.G.; Jack, A.S.; Child, J.A.; Morgan, G.J. Flow cytometric disease monitoring in multiple myeloma: The elationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood 2002, 100, 3095–3100. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.J.; Derman, B.A.; Bal, S.; Sidana, S.; Chhabra, S.; Silbermann, R.; Ye, J.C.; Cook, G.; Cornell, R.F.; Holstein, S.A.; et al. International harmonization in performing and reporting minimal residual disease assessment in multiple myeloma trials. Leukemia 2021, 35, 18–30. [Google Scholar] [CrossRef]
- Corradini, P.; Voena, C.; Tarella, C.; Astolfi, M.; Ladetto, M.; Palumbo, A.; Van Lint, M.T.; Bacigalupo, A.; Santoro, A.; Musso, M.; et al. Molecular and clinical remissions in multiple myeloma: Role of autologous and allogeneic transplantation of hematopoietic cells. J. Clin. Oncol. 1999, 17, 208–215. [Google Scholar] [CrossRef]
- Corradini, P.; Cavo, M.; Lokhorst, H.; Martinelli, G.; Terragna, C.; Majolino, I.; Valagussa, P.; Boccadoro, M.; Samson, D.; Bacigalupo, A.; et al. Molecular remission after myeloablative allogeneic stem cell transplantation predicts a better relapse-free survival in patients with multiple myeloma. Blood 2003, 102, 1927–1929. [Google Scholar] [CrossRef] [Green Version]
- Ladetto, M.; Ferrero, S.; Drandi, D.; Festuccia, M.; Patriarca, F.; Mordini, N.; Cena, S.; Benedetto, R.; Guarona, G.; Ferrando, F.; et al. Prospective molecular monitoring of minimal residual disease after non-myeloablative allografting in newly diagnosed multiple myeloma. Leukemia 2016, 30, 1211–1214. [Google Scholar] [CrossRef] [Green Version]
- Garfall, A.L.; Maus, M.V.; Hwang, W.T.; Lacey, S.F.; Mahnke, Y.D.; Melenhorst, J.J.; Zheng, Z.; Vogl, D.T.; Cohen, A.D.; Weiss, B.M.; et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N. Engl. J. Med. 2015, 373, 1040–1047. [Google Scholar] [CrossRef]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef] [Green Version]
- Raje, N.; Berdeja, J.; Lin, Y.; Chhabra, S.; Silbermann, R.; Ye, J.C.; Cook, G.; Cornell, R.F.; Holstein, S.A.; Shi, Q.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. “Off-the-shelf” allogeneic CAR T cells: Development and challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef]
- Gagelmann, N.; Riecken, K.; Wolschke, C.; Berger, C.; Ayuk, F.A.; Fehse, B.; Kröger, N. Development of CAR-T cell therapies for multiple myeloma. Leukemia 2020, 34, 2317–2332. [Google Scholar] [CrossRef]
- Wagner, V.; Gil, J. T cells engineered to target senescence. Nature 2020, 583, 37–38. [Google Scholar] [CrossRef]

| Reference | Conditioning | Transplant-Related Mortality % | Complete Remission % | Overall Survival % |
|---|---|---|---|---|
| 11 | Mel (100 mg/m2), TBI (12 Gy) | 53 (at 1 year) | --- | 39 (at 7 years) |
| 12 | Bu, Cy, ±TBI | 48 (at day 100) 63 (at 1 year) | 34 | 22 (at 5 years) |
| 13 | Cy, TBI Bu, Cy Mel (100 mg/m2), TBI | 19 (at day 100) | 62 | 47 (at 3 years) |
| 14 | Cy, TBI (14Gy) Bu, Cy | 10 | --- | 55 (at 2 years) |
| 15 | Mel (110 mg/m2), TBI (10.5Gy) Cy, TBI Cy, Mel Bu, Cy | 54 | 37 | 36 (at 3 years) |
| 16 | Cy, TBI (12Gy) Mel (140 mg/m2), TBI (10.5Gy) Bu, Cy Others | 22 | 57 | 32 (at 40 months) |
| 17 | Mel (160 mg/m2), TBI (12Gy) Cy, TBI (12Gy) Bu, Cy | 59 | 50 | 32 (at 3 years) |
| 18 | Cy, TBI Mel, TBI Bu, Cy, TBI Others | 25 | --- | 40 (at 3 years) |
| Reference | Type of Conditioning/Study Design | Transplant-Related Mortality | Event Free Survival or Progression-Free Survival | Overall Survival |
|---|---|---|---|---|
| [21] | Non-myeloablative/Prospective Phase II Auto-Allo in Newly Diagnosed MM | 0% at 100 days | NR | 78% at 552 days |
| [22] | Reduced-Intensity/Prospective Phase II Auto-Allo in Newly Diagnosed MM | 11% at 100 days | 70% at 13 months | 76% at 13 months |
| [23] | Reduced Intensity/Prospective Phase II Auto-Allo from Unrelated Donors | 10% at 100 days | 53% at 2 years | 74% at 2 years |
| [24,25] | Reduced Intensity/Prospective Comparison Auto-Allo vs. Tandem Auto in High Risk Newly Diagnosed MM | 11% vs. NR | 19 vs. 22 months | 34 vs. 49 months |
| [26,27] | Non-myeloablative/Prospective Randomised Auto-Allo vs. Tandem Auto in Newly Diagnosed MM | 16% vs. 2% at 6.5 years | 35 vs. 29 months | 80 vs. 54 months |
| [28] | Reduced Intensity/Prospective Comparison Auto-Allo vs. Tandem Auto in High Risk Newly Diagnosed MM | 16% vs. 5% | Not reached vs. 31 months | Not reached vs. 58 months |
| [29,30] | Non-myeloablative/Prospective Randomised Auto-Allo vs. Tandem Auto in Newly Diagnosed MM | 16% vs. 3% at 8 years | 25% vs. 18% at 8 years 27% vs. 15% at 8 years | 42% vs. 33% at 10 years 42% vs. 29% at 10 years |
| [31,32] | Non-myeloablative/Prospective Randomised Auto-Allo vs. Tandem Auto in Newly Diagnosed MM | 20% vs. 9% at 6 years 20% vs. 11% at 10 years | 22% vs. 25% at 6 years 18% vs. 19% at 10 years | 59% vs. 60% at 6 years 44% vs. 43% at 10 years |
| [33,34] | Non-myeloablative/Prospective Randomised Auto-Allo vs. Tandem Auto in Newly Diagnosed MM | NR | 43% vs. 39% at 3 years 22% vs. 12% at 8 years | 75% vs. 68% at 3 years 49% vs. 36% at 8 years 48% vs. 27% at 10 years |
| [35] | Multiple regimens/Retrospective Haploidentical Allografting in MM Relapsed/Refractory Patients | 10% at 18 months | 33% at 18 months | 63% at 18 months |
| [36] | Multiple regimens/Retrospective Haploidentical Allografting in MM Relapsed/Refractory Patients | 21% at 1 year | 17% at 2 year | 48% at 2 year |
| [37] | Multiple regimens/Prospective Randomised Auto-Allo vs. Tandem Auto in MM Patients in First Relapse | 27% at 5 years | NR | 31% vs. 9% at 7 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, B.; Lia, G.; Bonifazi, F.; Giaccone, L. Decades of Progress in Allogeneic Stem Cell Transplantation for Multiple Myeloma. Hemato 2021, 2, 89-102. https://0-doi-org.brum.beds.ac.uk/10.3390/hemato2010005
Bruno B, Lia G, Bonifazi F, Giaccone L. Decades of Progress in Allogeneic Stem Cell Transplantation for Multiple Myeloma. Hemato. 2021; 2(1):89-102. https://0-doi-org.brum.beds.ac.uk/10.3390/hemato2010005
Chicago/Turabian StyleBruno, Benedetto, Giuseppe Lia, Francesca Bonifazi, and Luisa Giaccone. 2021. "Decades of Progress in Allogeneic Stem Cell Transplantation for Multiple Myeloma" Hemato 2, no. 1: 89-102. https://0-doi-org.brum.beds.ac.uk/10.3390/hemato2010005






