Transmission of Soil Transmitted Helminthiasis in the Mifi Health District (West Region, Cameroon): Low Endemicity but Still Prevailing Risk
Abstract
:1. Introduction
2. Results
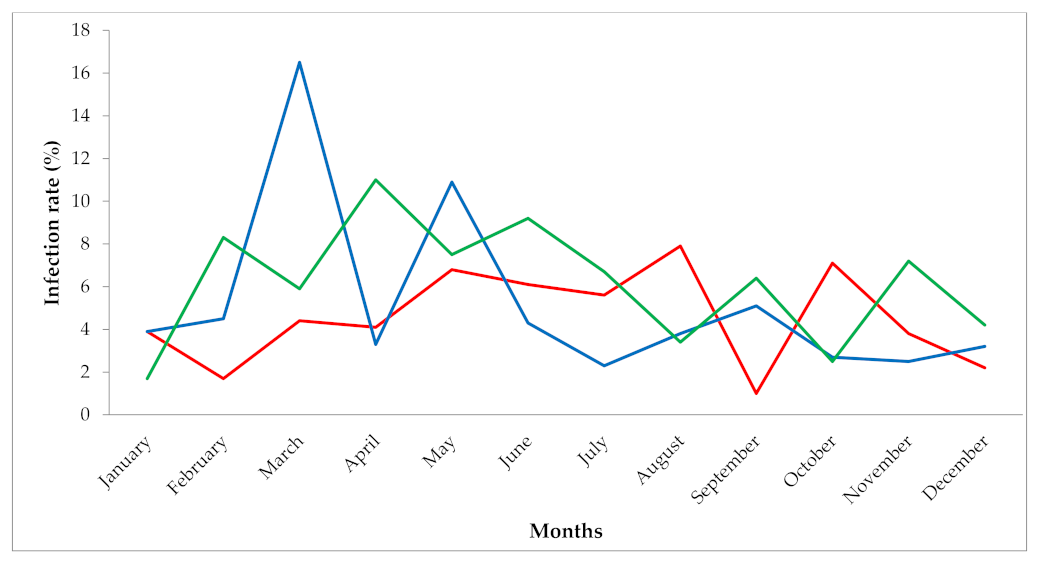
2.1. Retrospective Survey in Health Facilities
2.2. Prospective Survey in the Environment
2.3. Comparison between Infestation Rates in Health Facilities and Contamination Rates of the Environment
3. Discussion
4. Materials and Methods
4.1. Study Area and Population
4.2. Study Design
4.3. Soil Samples Collection and Processing
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Soil-Transmitted Helminth Infections: Key Facts; Updated 2 March 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 11 June 2021).
- Tchuem Tchuenté, L.A. Control of soil-transmitted helminths in sub-Saharan Africa: Diagnosis, drug efficacy concerns and challenges. Acta Trop. 2011, 120 (Suppl. S1), S4–S11. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [Green Version]
- Ratard, R.C.; Kouemeni, L.E.; Ekani Bessala, M.M.; Ndamkou, C.N.; Sama, M.M.; Cline, B.L. Ascariasis and trichuriasis in Cameroon. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 84–88. [Google Scholar] [CrossRef]
- Ratard, R.C.; Kouemeni, L.E.; Ekani Bessala, M.K.; Ndamkou, C.N. Distribution of hookworm infection in Cameroon. Ann. Trop. Med. Parasitol. 1992, 86, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Tchuem Tchuente, L.A.; N’Goran, E.K. Schistosomiasis and soil-transmitted helminthiasis control in Cameroon and Cote d’Ivoire: Implementing control on a limited budget. Parasitology 2009, 136, 1739–1745. [Google Scholar] [CrossRef]
- World Health Organization. Soil-Transmitted Helminthiasis—Eliminating Soil-Transmitted Helminthiases as a Public Health PROBLEM in Children. Soil-Transmitted Helminthiases Progress Report 2001–2010 and Strategic Plan 2011–2020; WHO: Geneva, Swizterland, 2012. [Google Scholar]
- World Health Organization. Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in at-Risk Population Groups; WHO: Geneva, Swizterland, 2017. [Google Scholar]
- Tchuem Tchuenté, L.A.; Kamwa Ngassam, R.I.; Sumo, L.; Ngassam, P.; Dongmo Noumedem, C.; LuogbouNzu, D.O.; Dankoni, E.; Kenfack, C.M.; Feussom Gipwe, N.; Akame, J.; et al. Mapping of schistosomiasis and soil-transmitted helminthiasis in the Regions of Centre, East and West Cameroon. PLoS Neg. Trop. Dis. 2012, 6, e1553. [Google Scholar] [CrossRef]
- Tchuem Tchuenté, L.A.; Dongmo Noumedem, C.; Ngassam, P.; Kenfack, C.M.; Feussom Gipwe, N.; Dankoni, E.; Tarini, A.; Zhang, Y. Mapping of schistosomiasis and soil-transmitted helminthiasis in the regions of Littoral, North-West, South and South-West Cameroon and recommendations for treatment. BMC Inf. Dis. 2013, 13, 602. Available online: http://0-www-biomedcentral-com.brum.beds.ac.uk/1471-2334/13/602 (accessed on 20 June 2021). [CrossRef] [Green Version]
- Djune-Yemeli, L.; Nana-Djeunga, H.C.; Lenou-Nanga, C.G.; Donfo-Azafack, C.; Domche, A.; Fossuo-Thotchum, F.; Niamsi-Emalio, Y.; Ntoumi, F.; Kamgno, J. Serious limitations of the current strategy to control Soil-Transmitted Helminths and added value of Ivermectin/Albendazole mass administration: A population-based observational study in Cameroon. PLoS Negl. Trop. Dis. 2020, 14, e0008794. [Google Scholar] [CrossRef]
- Jia, T.W.; Melville, S.; Utzinger, J.; King, C.H.; Zhou, X.N. Soil-transmitted helminth reinfection after drug treatment: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2012, 6, e1621. [Google Scholar] [CrossRef] [Green Version]
- Al-Mekhlafi, M.H.; Surin, J.; Atiya, A.S.; Ariffin, W.A.; Mahdy, M.A.K.; Abdullah, H.C. Pattern and predictors of soil-transmitted helminth reinfection among aboriginal schoolchildren in rural Peninsular Malaysia. Acta Trop. 2008, 107, 200–204. [Google Scholar] [CrossRef]
- Zerihun, Z.; Tsegaye, Y.; Befikadu, T. Soil-Transmitted Helminth Reinfection and Associated Risk Factors among School-Age Children in Chencha District, Southern Ethiopia: A Cross-Sectional Study. J. Paras. Res. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Nordin, A.; Nyberg, K.; Vinneras, B. Inactivation of Ascaris Eggs in Source-Separated Urine and Feces by Ammonia at Ambient Temperatures. App. Environ. Microbiol. 2009, 75, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngatou, T.; Nkouayep, V.R.; Pone, J.W. Soil Contamination Rate, Prevalence, Intensity of Infection of Geohelminths and Associated Risk Factors among Residents in Bazou (West Cameroon). EJHS 2018, 28, 63–72. [Google Scholar]
- Programme National de Lutte contre la Schistosomiase et les Helminthiases Intestinales au Cameroun. Plan Strat. 2005, 2005–2010.
- Ntonifor, N.H.; Sumbele, N.I.; Tabot, E.J. Soil-Transmitted Helminth Infection and Associated risk factors in a neglected region in the upper Nkongho-mbo area, South-West Region Cameroon. Int. J. Trop. Dis. Health 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Shrastha, A.; Basnyat, S.R.; Skakya, B.; Rai, S.K.; Rai, C.; Rai, C.K. Soil Transmitted helminthiasis in kathamandu, Nepal Katmandu University. Med. J. 2007, 9, 166–169. [Google Scholar]
- Steinbaum, L.; Kwong, L.H.; Ercumen, A.; Makeda, S.N.; Amira, J.L.; Njenga, M.S.; Boehm, A.B.; Pickering, A.J.; Kara, L.N. Detecting and enumerating soil transmitted Helminths eggs in soil. New method development and results from field testing in kenya and Bangladesh. PLoS Negl. Trop. Dis. 2017, 11, e0005522. [Google Scholar] [CrossRef] [Green Version]
- Mbub, J.V.; Ntonifor, H.N.; Ojong, J. The epidemiology of soil transmitted helminths and protozoan in south-west Cameroon. J. Hel. 2012, 86, 30–37. [Google Scholar]
- Vaz Nery, S.; Pickering, A.J.; Abate, E.; Asmare, A.; Barrett, L.; Benjamin-Chung, J.; Bundy, D.A.P.; Clasen, T.; Clements, A.C.A.; Colford, J.M., Jr.; et al. The role of water, sanitation and hygiene interventions in reducing soil-transmitted helminths: Interpreting the evidence and identifying next steps. Parasit Vectors 2019, 12, 273. [Google Scholar] [CrossRef]
- Tabi, E.S.; Eyong, E.M.; Akum, E.A.; Love, J.; Cumber, S.N. Soil-transmitted helminths infections in Tiko Health Distric, South West Region of Cameroon: A post-intervention survey on prevalence and intensity of infection among primary school children. Pan. Afr. Med. J. 2018, 30, 74–82. [Google Scholar] [PubMed]
- Tembo, S.; Mubita, P.; Sitali, L.; Zgambo, J. Prevalence, Intensity, and Factors Associated with Soil-Transmitted Helminths Infection among Children in Zambia: A Cross-sectional Study. Bentham Open 2019, 12. [Google Scholar] [CrossRef]
- Smyth, J.D. Animal parasitology. Cambridge: Cambridge University Press. Low Price Edition. Trans. Roy. Soc. Trop Med. Hyg 1996, 91, 95. [Google Scholar] [CrossRef]
- National Atlas of Physical Development of Cameroon. 2013. Available online: https://fr.slideshare.net/ninonjopkou/positionnement-gographique-des-activits-conomiques-du-cameroun (accessed on 5 May 2021).
- World Health Organization. Health Population Denominators 2017-Cameroon; WHO: Yaounde, Cameroon, 2017; p. 98. [Google Scholar]
- World Health Organization. Basic Laboratory Methods in Parasitology; WHO Press: Geneva, Swizterland, 1991. [Google Scholar]
- Tavalla, M.; Oormazdi, H.; Akhlaghi, L.; Razmiou, E.; Lakeh, M.M.; Shojaee, S.; Hadighi, R.; Meamar, R.A. Prevalence of parasites in soil samples in Teheran public places. Afr. J. Biotech. 2012, 11, 4575–4578. [Google Scholar]
- Thienpont, D.; Rochett, F.; Vanparijs, O. Coprological Diagnosis of Helminthosis; Janssen Research Foundation: Beerse, Belgium, 1979; pp. 1–187. [Google Scholar]
- Soulsby, E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals, 7th ed.; Baillere: London, UK, 1982; pp. 1–809. [Google Scholar]

| Characteristics | No. Patients Examined | No. Patients Infected | Infestation Rate (95% CI) | Chi-Square (p-Value) |
|---|---|---|---|---|
| Gender | 0.290 (0.592) | |||
| Female | 3307 | 159 | 4.8 (4.1–5.6) | |
| Male | 1549 | 80 | 5.2 (4.2–6.4) | |
| Age groups (years) | 65.056 (0.0001) | |||
| ≤10 | 1444 | 105 | 7.3 (6.0–8.7) | |
| (11–20) | 863 | 56 | 6.5 (5.0–8.3) | |
| (21–30) | 975 | 13 | 1.3 (0.8–2.3) | |
| (31–50) | 878 | 20 | 2.3 (1.5–3.5) | |
| ≥51 | 696 | 45 | 6.5 (4.9–8.5) | |
| Health facilities | 1205.622 (0.0001) | |||
| Badiembou | 324 | 52 | 16 (12.5–20.4) | |
| Bapi | 538 | 179 | 33.3 (29.4–37.4) | |
| Djeleng | 3179 | 1 | 0 (0.0–0.2) | |
| Famchouet | 176 | 7 | 4 (1.9–8.0) | |
| Kongso | 639 | 0 | 0 (0.0–0.6) | |
| Years | 4.066 (0.131) | |||
| 2018 | 1634 | 72 | 4.4 (3.5–5.5) | |
| 2019 | 2076 | 98 | 4.7 (3.9–5.7) | |
| 2020 | 1146 | 69 | 6 (4.8–7.6) | |
| Total | 4856 | 239 | 4.9 |
| Sampling Biotopes | No. Samples Examined | N with Ascaris (%) | N with Trichuris (%) | N with STH (%) |
|---|---|---|---|---|
| Dustbins | 40 | 4(10) | 0 | 4(10) |
| Markets | 40 | 6(15) | 0 | 6(15) |
| Roads | 40 | 1(2.5) | 0 | 1(2.5) |
| Schools | 40 | 5(12.5) | 1(2.5) | 6(15) |
| Houses | 40 | 4(10) | 3(7.5) | 7(17.5) |
| Overall | 200 | 20(10) | 4(2) | 24(12) |
| Community | Sites | N Examined | No Positives (%) | Chi-Square | p-Value |
|---|---|---|---|---|---|
| Badiembou | Health facility | 121 | 23 (19.0) | 0.23 | 0.6315 |
| Environment | 40 | 9 (22.5) | |||
| Bapi | Health facility | 122 | 44 (36.1) | 11.94 | 0.0005 |
| Environment | 40 | 3 (7.5) | |||
| Djeleng | Health facility | 614 | 0 (0.0) | Fisher exact test | 0.0612 |
| Environment | 40 | 1 (2.5) | |||
| Famtchouet | Health facility | 84 | 2 (2.4) | Fisher exact test | 0.0136 |
| Environment | 40 | 6 (15.0) | |||
| Kongso | Health facility | 205 | 0 (0.0) | Fisher exact test | <0.0001 |
| Environment | 40 | 5 (12.5) | |||
| Overall | Health facility | 1146 | 69 (6.0) | 9.46 | 0.0021 |
| Environment | 200 | 24 (12.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumo, L.; Otiobo Atibita, E.N.; Mache, E.; Gangue, T.; Nana-Djeunga, H.C. Transmission of Soil Transmitted Helminthiasis in the Mifi Health District (West Region, Cameroon): Low Endemicity but Still Prevailing Risk. Parasitologia 2021, 1, 95-104. https://0-doi-org.brum.beds.ac.uk/10.3390/parasitologia1030011
Sumo L, Otiobo Atibita EN, Mache E, Gangue T, Nana-Djeunga HC. Transmission of Soil Transmitted Helminthiasis in the Mifi Health District (West Region, Cameroon): Low Endemicity but Still Prevailing Risk. Parasitologia. 2021; 1(3):95-104. https://0-doi-org.brum.beds.ac.uk/10.3390/parasitologia1030011
Chicago/Turabian StyleSumo, Laurentine, Esther Nadine Otiobo Atibita, Eveline Mache, Tiburce Gangue, and Hugues C. Nana-Djeunga. 2021. "Transmission of Soil Transmitted Helminthiasis in the Mifi Health District (West Region, Cameroon): Low Endemicity but Still Prevailing Risk" Parasitologia 1, no. 3: 95-104. https://0-doi-org.brum.beds.ac.uk/10.3390/parasitologia1030011





