Chemical Composition of Apples Cultivated in Norway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apples
2.2. Sample Preparation
Apple Juice Extraction and Sampling
2.3. Chemical Analyses
2.3.1. UV-MS Reversed Phase HPLC of Polyphenols Directly or after Phloroglucinolysis Reaction
2.3.2. Total Phenols (Folin Ciocalteu Assay)
2.3.3. Density (d20/20)
2.3.4. pH
2.3.5. Nitrogen
2.3.6. Free Amino Acids (FAA)
2.4. Statistical Analyses
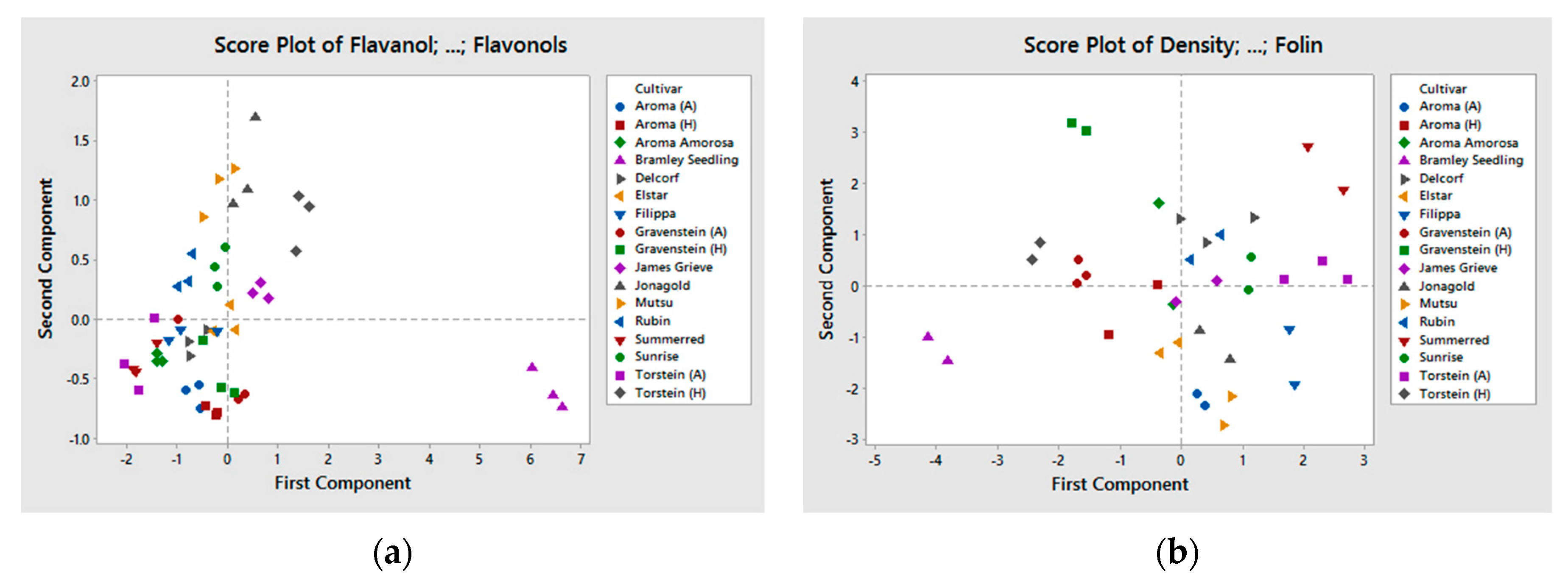
3. Results and Discussion
3.1. Apples
3.1.1. Chemical Composition of Apple Juice
3.1.2. Potential Use of Apples for Cider
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Alberti, A.; dos Santos, T.P.M.; Zielinski, A.A.F.; dos Santos, C.M.E.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. Lwt Food Sci. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Guyot, S.; Le Bourvellec, C.; Marnet, N.; Drilleau, J. Procyanidins are the most abundant polyphenols in dessert apples at maturity. Food Sci. Technol. 2002, 35, 289–291. [Google Scholar] [CrossRef]
- Lea, A. Craft Cider Making; The Good Life Press Ltd.: Preston, UK, 2010. [Google Scholar]
- Ewing, B.L.; Peck, G.M.; Ma, S.; Neilson, A.P.; Stewart, A.C. Management of Apple Maturity and Postharvest Storage Conditions to Increase Polyphenols in Cider. HortScience 2019, 54, 143–148. [Google Scholar] [CrossRef] [Green Version]
- BBai, X.; Zhang, H.; Ren, S. Antioxidant activity and HPLC analysis of polyphenol-enriched extracts from industrial apple pomace. J. Sci. Food Agric. 2013, 93, 2502–2506. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Psomas, A.; Zovoili, A.; Siatis, V. Polyphenolic profile and antioxidant activity of five apple cultivars grown under organic and conventional agricultural practices. Int. J. Food Sci. Technol. 2009, 44, 1167–1175. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Xie, S.; Sockovie, E.; Khanizadeh, S. Which Polyphenolic Compounds Contribute to the Total Antioxidant Activities of Apple? J. Agric. Food Chem. 2005, 53, 4989–4995. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; García-Villalba, R.; Tomás-Barberán, F.A. Polyphenolic characterisation of old local apple varieties from Southeastern European region. J. Food Compos. Anal. 2013, 31, 199–211. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.; Kraus, M.; Richling, E. Polyphenol profiles of apple juices. Mol. Nutr. Food Res. 2005, 49, 797–806. [Google Scholar] [CrossRef]
- Cheng, L.L.; Raba, R. Accumulation of Macro- and Micronutrients and Nitrogen Demand-supply Relationship of ’Gala’/’Malling 26’ Apple Trees Grown in Sand Culture. J. Am. Soc. Hortic. Sci. 2009, 134, 3–13. [Google Scholar] [CrossRef]
- Karl, A.D.; Brown, M.G.; Ma, S.; Sandbrook, A.; Stewart, A.C.; Cheng, L.; Mansfield, A.K.; Peck, G.M. Soil Nitrogen Fertilization Increases Yeast Assimilable Nitrogen Concentrations in ‘Golden Russet’ and ‘Medaille d’Or’ Apples Used for Cider Production. Hortscience 2020, 55, 1345–1355. [Google Scholar] [CrossRef]
- Ma, S.; Neilson, A.P.; Lahne, J.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free amino acid composition of apple juices with potential for cider making as determined by UPLC-PDA. J. Inst. Brew. 2018, 124, 467–476. [Google Scholar] [CrossRef]
- Boudreau, T.F.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free amino nitrogen concentration correlates to total yeast assimilable nitrogen concentration in apple juice. Food Sci. Nutr. 2018, 6, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, T.F.; Peck, G.M.; Ma, S.; Patrick, N.; Duncan, S.; O’Keefe, S.F.; Stewart, A.C. Hydrogen sulphide production during cider fermentation is moderated by pre-fermentation methionine addition. J. Inst. Brew. 2017, 123, 553–561. [Google Scholar] [CrossRef]
- Di Maro, A.; Dosi, R.; Ferrara, L.; Rocco, M.; Sepe, J.; Ferrari, G.; Parente, A. Free amino acid profile in Malus domestica cv Annurca apples from the Campania region and other Italian vegetables. Aust. J. Crop Sci. 2011, 5, 154–161. [Google Scholar]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Zuo, W.; Zhang, T.; Xu, H.; Wang, C.; Lu, M.; Chen, X.; Xu, L. Effect of fermentation time on nutritional components of red-fleshed apple cider. Food Bioprod. Process. 2019, 114, 276–285. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Changes in the profile of volatile compounds and amino acids during cider fermentation using dessert variety of apples. Eur. Food Res. Technol. 2014, 239, 67–77. [Google Scholar] [CrossRef]
- Villière, A.; Arvisenet, G.; Bauduin, R.; Le Quéré, J.-M.; Sérot, T. Influence of cider-making process parameters on the odourant volatile composition of hard ciders. J. Inst. Brew. 2015, 121, 95–105. [Google Scholar] [CrossRef]
- Vangdal, E.; Kvamm-Lichtenfeld, K. Ciders produced from Norwegian fresh consumption apple cultivars. Acta Hortic. 2018, 1205, 527–532. [Google Scholar] [CrossRef]
- Bradshaw, T.; Kingsley-Richards, S.; Foster, J. Apple cultivar evaluations for cider making in Vermont, USA. Acta Hortic. 2018, 1205, 453–460. [Google Scholar] [CrossRef]
- Nicolini, G.; Román, T.; Carlin, S.; Malacarne, M.; Nardin, T.; Bertoldi, D.; Larcher, R. Characterisation of single-variety still ciders produced with dessert apples in the Italian Alps. J. Inst. Brew. 2018, 124, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Le Bourvellec, C.; Le Quéré, J.-M.; Sanoner, P.; Drilleau, J.-F.; Guyot, S. Inhibition of Apple Polyphenol Oxidase Activity by Procyanidins and Polyphenol Oxidation Products. J. Agric. Food Chem. 2004, 52, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Malec, M.; Le Quéré, J.-M.; Sotin, H.; Kolodziejczyk, K.; Bauduin, R.; Guyot, S. Polyphenol Profiling of a Red-Fleshed Apple Cultivar and Evaluation of the Color Extractability and Stability in the Juice. J. Agric. Food Chem. 2014, 62, 6944–6954. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Jones, G.P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Moe, K.; Porcellato, D.; Skeie, S. Metabolism of milk fat globule membrane components by nonstarter lactic acid bacteria isolated from cheese. J. Dairy Sci. 2013, 96, 727–739. [Google Scholar] [CrossRef] [Green Version]
- Alexander, T.R.; King, J.; Zimmerman, A.; Miles, C.A. Regional Variation in Juice Quality Characteristics of Four Cider Apple (Malus xdomestica Borkh.) Cultivars in Northwest and Central Washington. Hortscience 2016, 51, 1498–1502. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Bureau, S.; Renard, C.M.G.C.; Plenet, D.; Gautier, H.; Touloumet, L.; Girard, T.; Simon, S. Cultivar and Year Rather than Agricultural Practices Affect Primary and Secondary Metabolites in Apple Fruit. PLoS ONE 2015, 10, e0141916. [Google Scholar] [CrossRef]
- Thompson-Witrick, K.A.; Goodrich, K.M.; Neilson, A.P.; Hurley, E.K.; Peck, G.M.; Stewart, A.C. Characterization of the Polyphenol Composition of 20 Cultivars of Cider, Processing, and Dessert Apples (Malus×domesticaBorkh.) Grown in Virginia. J. Agric. Food Chem. 2014, 62, 10181–10191. [Google Scholar] [CrossRef]
- Wrona, D. The influence of nitrogen fertilization on growth, yield and fruit size of ‘Jonagored’apple trees. Acta Sci. Pol. Hortorum Cultus 2011, 10, 3–10. [Google Scholar]
- Nava, G.; Dechen, A.R. Long-term annual fertilization with nitrogen and potassium affect yield and mineral composition of ‘fuji’ apple. Sci. Agric. 2009, 66, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Ernani, P.R.; Rogeri, D.A.; Proença, M.M.; Dias, J. Addition of nitrogen had no effect on yield and quality of apples in an high density orchard carrying a dwarf rootstock. Rev. Bras. Frutic. 2008, 30, 1113–1118. [Google Scholar] [CrossRef] [Green Version]
- Lea, A.G.H.; Drilleau, J.-F. Fermented Beverage Production; Kluwer Academic: New York, NY, USA, 2003. [Google Scholar]
- Eleutério dos Santos, C.M.; Pietrowski, G.D.A.M.; Braga, C.M.; Rossi, M.J.; Ninow, J.; Machado dos Santos, T.P.; Wosiacki, G.; Matos Jorge, R.M.; Nogueira, A. Apple Aminoacid Profile and Yeast Strains in the Formation of Fusel Alcohols and Esters in Cider Production. J. Food Sci. 2015, 80, C1170–C1177. [Google Scholar] [CrossRef]
- Jolicoeur, C. The New Cider Makers Handbook; Chelsea Green Publishing: White River Junction, Windsor, VT, USA, 2013. [Google Scholar]
- Guyot, S.; Marnet, N.; Sanoner, P.; Drilleau, J.-F. Variability of the Polyphenolic Composition of Cider Apple (Malus domestica) Fruits and Juices. J. Agric. Food Chem. 2003, 51, 6240–6247. [Google Scholar] [CrossRef] [PubMed]
- Francini, A.; Sebastiani, L. Phenolic Compounds in Apple (Malus x domestica Borkh.): Compounds Characterization and Stability during Postharvest and after Processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastasiadi, M.; Mohareb, F.; Redfern, S.P.; Berry, M.; Simmonds, M.S.J.; Terry, L.A. Biochemical Profile of Heritage and Modern Apple Cultivars and Application of Machine Learning Methods To Predict Usage, Age, and Harvest Season. J. Agric. Food Chem. 2017, 65, 5339–5356. [Google Scholar] [CrossRef] [PubMed]
- Verdu, C.F.; Childebrand, N.; Marnet, N.; LeBail, G.; Dupuis, F.; Laurens, F.; Guilet, D.; Guyot, S. Polyphenol variability in the fruits and juices of a cider apple progeny. J. Sci. Food Agric. 2014, 94, 1305–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Rosend, J.; Kuldjärv, R.; Rosenvald, S.; Paalme, T. The effects of apple variety, ripening stage, and yeast strain on the volatile composition of apple cider. Heliyon 2019, 5, e01953. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, A.; Le Quéré, J.M.; Gestin, P.; Michel, A.; Wosiacki, G.; Drilleau, J.F. Slow Fermentation in French Cider Processing due to Partial Biomass Reduction. J. Inst. Brew. 2008, 114, 102–110. [Google Scholar] [CrossRef]
- Symoneaux, R.; Chollet, S.; Bauduin, R.; Le Quéré, J.; Baron, A. Impact of apple procyanidins on sensory perception in model cider (part 2): Degree of polymerization and interactions with the matrix components. Lwt Food Sci. Technol. 2014, 57, 28–34. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Han, Y. Quantification of Volatile Compoundsin Chinese Ciders by Stir Bar Sorptive Extraction (SBSE) and Gas Chromatography—Mass Spectrometry (GC–MS). J. Inst. Brew. 2011, 117, 61–66. [Google Scholar] [CrossRef]
- Denby, C.M.; Li, R.A.; Vu, V.T.; Costello, Z.; Lin, W.; Chan, L.J.G.; Williams, J.; Donaldson, B.; Bamforth, C.W.; Petzold, C.J.; et al. Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
| Cultivar | Origin | Parentage | Age * | Growing Location (Norway) ** |
|---|---|---|---|---|
| Aroma Amorosa | Sweden | Ingrid Marie/Filippa | New | A |
| Aroma | Sweden 1973 | Ingrid Marie/Filippa | New | A + H |
| Bramley Seedling | England 1837 | Unknown | Old | H |
| Delcorf (Estivale) | France 1950 | Stark Jongrimes/Golden Delicious | New | A |
| Elstar | Holland 1972 | Golden Delicious/Ingrid Marie | Old | A |
| Filippa | Denmark from 1880 | Unknown | Old | A |
| Gravenstein | Denmark from 1669. in Norway from 1792 | Unknown | Old | A + H |
| James Grieve | Scotland 1893 | Pott’s Seedling | New | H |
| Jonagold | New York. US. 1943 | Jonathan/Golden Delicious | Old | A |
| Mutsu | Japan 1930 | Golden Delicious/Indo | Old | A |
| Rubin | Czech Republic 1960 | Golden Delicious/Lord Lambourne | New | A |
| Sunrise | Canada 1990 | McIntosh/Golden Delicious | New | A |
| Summerred | Canada 1964 | McIntosh/Golden Delicious | Old | A |
| Torstein red | Derived from Torstein | Old | A | |
| Torstein | Unknown (Norway 1760) | Unknown | Old | H |
| Cultivar | Fruit Weight | Density (Juice) | pH |
|---|---|---|---|
| g ± STD | g/L ± STD | ||
| Aroma Amorosa | 155 ± 4.2 d | 1053 ± 11.9 b,c,d,e,f | 3.2 ± 0.01 c |
| Aroma (A) | 178 ± 5.3 e | 1058 ± 3.3 e,f | 3.2 ± 0.01 c |
| Aroma (H) | 286 ± 9.3 h | 1051 ± 3.6 d,e | 3.2 ± 0.14 c,d |
| Bramley Seedling | 135 ± 5.5 c | 1046 ± 10.8 a,b,c,d,e | 2.9 ± 0.11 a |
| Delcorf | 235 ± 6.7 g | 1050 ± 0.6 d | 3.3 ± 0.01 d,e |
| Elstar | 119 ± 3.1 b | 1053 ± 1.2 e | 3.1 ± 0.04 b |
| Filippa | 200 ± 5.5 f | 1051 ± 4.0 c,d,e | 3.1 ± 0.08 b |
| Gravenstein (A) | 164 ± 6.2 d | 1050 ± 0.3 d | 3.3 ± 0.02 d |
| Gravenstein (H) | 115 ± 3.4 b | 1034 ± 0.7 a | 3.2 ± 0.02 c |
| James Grieve | 116 ± 3.0 b | 1046 ± 1.9 c | 3.2 ± 0.00 c |
| Jonagold | 181 ± 3.2 e | 1055 ± 3.5 e | 3.4 ± 0.14 d,e |
| Mutsu | 215 ± 8.0 f | 1060 ± 1.5 f | 3.3 ± 0.06 d,e |
| Rubin | 191 ± 4.5 e | 1053 ± 1.4 e | 3.3 ± 0.04 c,d |
| Sunrise | 162 ± 2.8 d | 1044 ± 1.2 b | 3.4 ± 0.04 e |
| Summerred | 124 ± 4.4 b | 1039 ± 2.1 b | 3.3 ± 0.05 c,d |
| Torstein red (A) | 123 ± 3.1 b | 1049 ± 0.7 d | 3.2 ± 0.07 b,c |
| Torstein (H) | 80 ± 2.6 a | 1047 ± 0.9 c,d | 3.2 ± 0.02 b,c |
| Average | 164 | 1049 | 3.2 |
| Nitrogen | Sum FAA | ASN | ASP | GLU | SER | ALA | GABA | PHE | MET | |
|---|---|---|---|---|---|---|---|---|---|---|
| mg N/L ± STD | mg/kg ± STD | % | % | % | % | % | % | % | % | |
| Aroma Amorosa | 82.7 ± 6.3 b | 289.5 ± 0.2 d | 52.8 | 24.9 | 12.8 | 2.3 | 1.1 | 0.9 | 0.1 | 0.1 |
| Aroma (A) | 86.5 ± 0.4 b | 249.7 ± 0.2 c,d | 48.1 | 24.9 | 14.5 | 2.9 | 1.5 | 1.4 | 0.2 | 0.1 |
| Aroma (H) | 107.9 ± 23.8 b,c | 391.4 ± 0.8 d,e | 53.0 | 24.9 | 13.8 | 2.1 | 1.0 | 0.9 | 0.1 | 0.1 |
| Bramley Seedling | 74.9 ± 2.7 b | 253.4 ± 0.3 c,d | 37.5 | 31.4 | 20.8 | 1.9 | 1.2 | 1.6 | 0.2 | 0.1 |
| Delcorf | 135.8 ± 19.2 c,d | 455.3 ± 0.8 d,e | 49.7 | 13.8 | 6.1 | 9.5 | 10.8 | 1.6 | 0.1 | 0.2 |
| Elstar | 82.8 ± 7.7 b | 273.4 ± 0.4 d | 32.7 | 31.6 | 23.2 | 2.4 | 1.9 | 1.3 | 0.2 | ND |
| Filippa | 146.8 ± 1.0 d | 683.2 ± 0.5 e | 69.7 | 8.9 | 6.2 | 3.8 | 2.9 | 0.6 | 0.1 | 0.1 |
| Gravenstein (A) | 66.5 ± 3.8 a,b | 173.7 ± 0.2 b | 40.2 | 32.6 | 15.9 | 2.1 | 3.4 | 0.4 | 0.0 | ND |
| Gravenstein (H) | 77.2 ± 6.6 b | 213.6 ± 0.1 c | 47.7 | 25.5 | 16.0 | 1.7 | 2.2 | 0.7 | 0.1 | ND |
| James Grieve | 117.7 ± 10.6 c,d | 380.4 ± 0.4 d,e | 57.8 | 23.5 | 9.7 | 2.1 | 1.1 | 0.7 | 0.3 | 0.3 |
| Jonagold | 103.7 ± 4.1 b,c | 359.5 ± 0.4 d,e | 55.1 | 19.9 | 15.3 | 1.4 | 1.3 | 0.8 | 0.4 | 0.1 |
| Mutsu | 82.8 ± 5.6 b | 301.2 ± 0.4 d | 47.2 | 21.5 | 17.5 | 3.6 | 1.8 | 0.8 | 0.3 | 0.3 |
| Rubin | 125.8 ± 16.6 c,d | 446.2 ± 0.8 d,e | 50.7 | 18.6 | 13.7 | 7.0 | 2.0 | 1.4 | 0.1 | 0.4 |
| Sunrise | 131.0 ± 11.8 c,d | 459.9 ± 0.5 d,e | 40.7 | 37.2 | 8.5 | 2.0 | 3.5 | 2.6 | 0.0 | 0.1 |
| Summerred | 171.4 ± 26.5 d,e | 924.5 ± 0.8 f | 46.0 | 44.8 | 3.7 | 0.9 | 0.8 | 1.0 | 0.1 | 0.1 |
| Torstein red (A) | 139.8 ± 5.8 c,d | 613.9 ± 1.2 e | 64.6 | 16.9 | 9.3 | 2.3 | 2.1 | 0.8 | 0.1 | 0.2 |
| Torstein (H) | 43.2 ± 2.4 a | 42.4 ± 0.0 a | 15.9 | 36.3 | 28.0 | 4.5 | 1.9 | 0.8 | 0.0 | ND |
| Average | 105 | 382.4 | 47.6 | 25.7 | 13.8 | 3.1 | 2.4 | 1.1 | 0.1 | 0.1 |
| TP (Folin) | TotalPolyphenols (HPLC) | TotalFlavanols 1 | DPn 2 | TotalHydroxycin-namic Acids 3 | TotalDihydrochalcones 4 | TotalFlavonols 5 | |
|---|---|---|---|---|---|---|---|
| mg/L EPI equiv ± STD | mg/L | mg/L | mg/L | mg/L | mg/L | ||
| Aroma Amorosa | 598 ± 31 c | 209 b,c | 110 | 2.3 | 86 | 10 | 3 |
| Aroma (A) | 705 ± 18 c,d | 425 d | 227 | 1.9 | 180 | 15 | 3 |
| Aroma (H) | 966 ± 47 f | 587 e | 348 | 1.4 | 225 | 12 | 3 |
| Bramley Seedling | 1934 ± 42 g | 1838 f | 1042 | 3.0 | 765 | 27 | 4 |
| Delcorf | 666 ± 47 c,d | 397 c,d | 271 | 2.6 | 109 | 13 | 4 |
| Elstar | 696 ± 46 c,d | 383 d | 244 | 2.2 | 102 | 32 | 5 |
| Filippa | 730 ± 131 c,d | 290 b,c,d | 68 | 3.3 | 204 | 14 | 4 |
| Gravenstein (A) | 922 ± 40 f | 525 d,e | 345 | 2.6 | 157 | 20 | 3 |
| Gravenstein (H) | 855 ± 3 e | 468 d | 186 | 2.8 | 245 | 33 | 4 |
| James Grieve | 850 ± 12 e | 483 d,e | 302 | 2.1 | 136 | 38 | 7 |
| Jonagold | 796 ± 21 e | 370 d | 197 | 2.6 | 141 | 22 | 10 |
| Mutsu | 679 ± 24 c,d | 281 b,c | 113 | 2.8 | 144 | 15 | 8 |
| Rubin | 624 ± 26 c | 260 c,d | 172 | 2.4 | 70 | 12 | 5 |
| Sunrise | 658 ± 53 c,d | 371 d | 271 | 2.2 | 73 | 22 | 6 |
| Summerred | 272 ± 18 a | 87 a | 34 | 9.5 | 39 | 13 | 1 |
| Torstein red (A) | 453 ± 65 b | 87 a,b | 13 | 1.0 | 67 | 6 | 2 |
| Torstein (H) | 990 ± 37 f | 693 e | 354 | 1.9 | 299 | 29 | 10 |
| Average | 775 | 450 | 246 | 2.7 | 179 | 20 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wicklund, T.; Guyot, S.; Le Quéré, J.-M. Chemical Composition of Apples Cultivated in Norway. Crops 2021, 1, 8-19. https://0-doi-org.brum.beds.ac.uk/10.3390/crops1010003
Wicklund T, Guyot S, Le Quéré J-M. Chemical Composition of Apples Cultivated in Norway. Crops. 2021; 1(1):8-19. https://0-doi-org.brum.beds.ac.uk/10.3390/crops1010003
Chicago/Turabian StyleWicklund, Trude, Sylvain Guyot, and Jean-Michel Le Quéré. 2021. "Chemical Composition of Apples Cultivated in Norway" Crops 1, no. 1: 8-19. https://0-doi-org.brum.beds.ac.uk/10.3390/crops1010003






