Density, Enthalpy of Vaporization and Local Structure of Neat N-Alkane Liquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Simulation Methods
2.1.1. Liquid Simulation Details and Protocol
2.1.2. Gas Simulation Details and Protocol
2.2. Analysis of Simulations
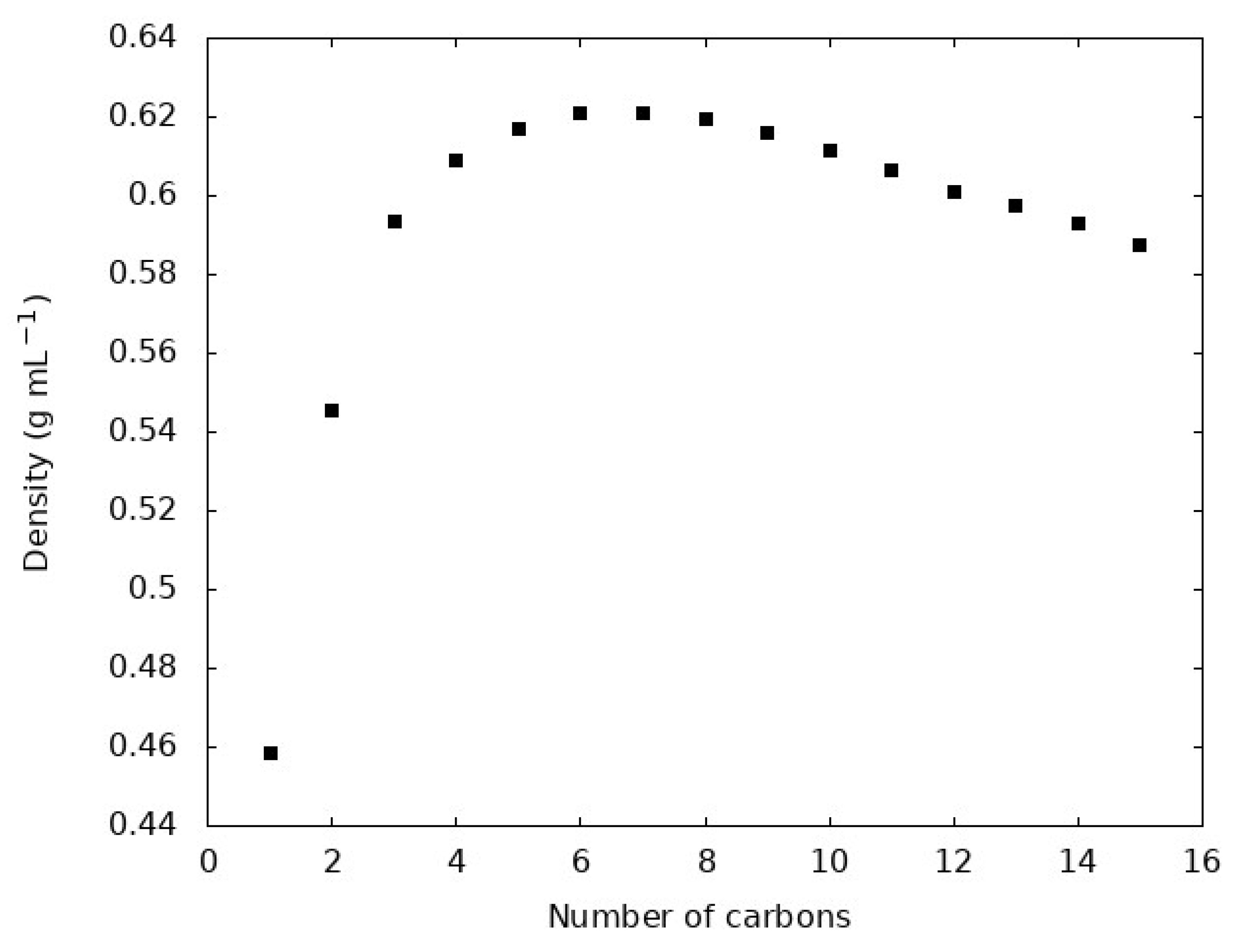
2.2.1. Density
2.2.2. Enthalpy of Vaporization ()
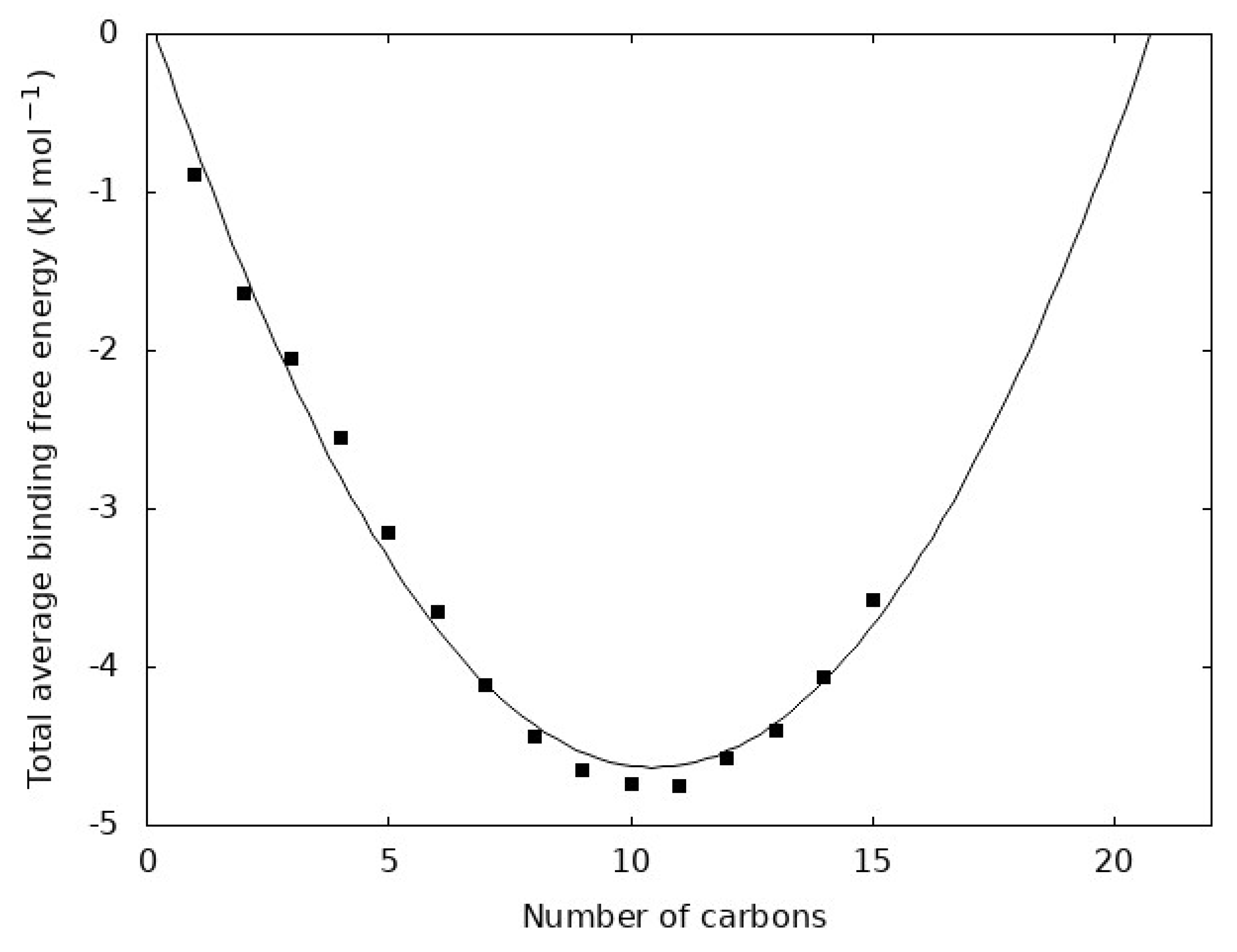
2.2.3. Radial Distribution Functions, Free Energy of Binding, and Entropy of Binding
3. Results
4. Discussion
4.1. Density, , and Evaluation of the GAFF2 Force Field for N-Alkanes
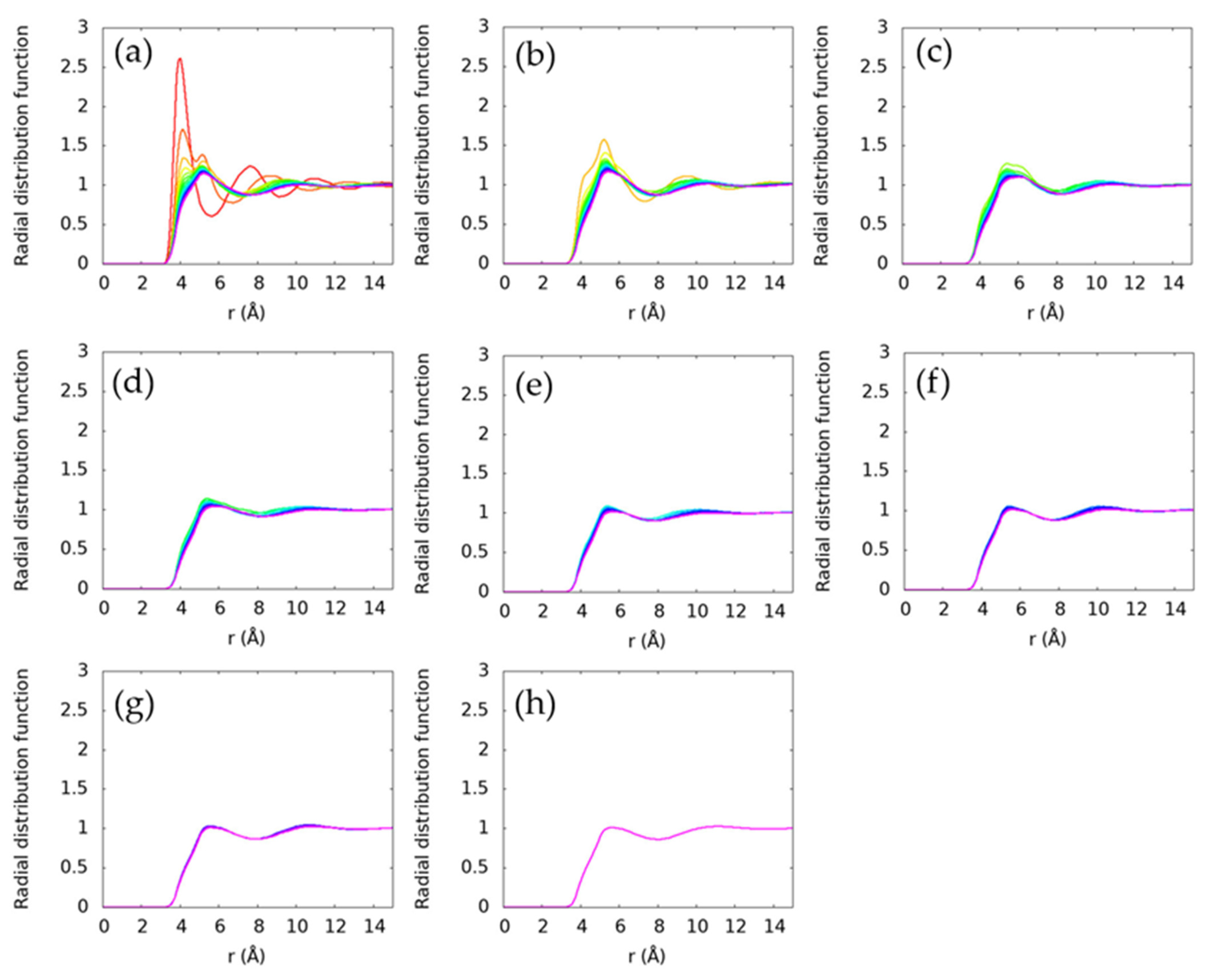
4.2. Average Structure in N-Alkane Liquids
4.3. Local Structure in N-Alkane Liquids
4.4. Thermodynamics of Binding
4.5. Consideration of Longer N-Alkanes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Freund, M.; Csikos, R.; Keszthelyi, S.; Mozes, G. Paraffin Products; Properties, Technologies, Applications; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Mango, F.D. The Light Hydrocarbons in Petroleum: A Critical Review. Org. Geochem. 1997, 26, 417–440. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Jackson, R.B.; Saunois, M.; Bousquet, P.; Canadell, J.G.; Poulter, B.; Stavert, A.R.; Bergamaschi, P.; Niwa, Y.; Segers, A.; Tsuruta, A. Increasing Anthropogenic Methane Emissions Arise Equally from Agricultural and Fossil Fuel Sources. Environ. Res. Lett. 2020, 15, 071002. [Google Scholar] [CrossRef]
- Cruikshank, D.P.; Pendleton, Y.J.; Grundy, W.M. Organic Components of Small Bodies in the Outer Solar System: Some Results of the New Horizons Mission. Life 2020, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, S.M.; Birch, S.P.D.; Horst, S.; Sotin, C.; Barth, E.; Lora, J.M.; Trainer, M.G.; Corlies, P.; Malaska, M.J.; Sciamma-O’Brien, E.; et al. Titan: Earth-like on the Outside, Ocean World on the Inside. Planet. Sci. J. 2021, 2, 112. [Google Scholar] [CrossRef]
- Engle, A.E.; Hanley, J.; Dustrud, S.; Thompson, G.; Lindberg, G.E.; Grundy, W.M.; Tegler, S.C. Phase Diagram for the Methane-Ethane System and Its Implications for Titan’s Lakes. Planet. Sci. J. 2021, 2, 118. [Google Scholar] [CrossRef]
- Hillyer, M.B.; Gibb, B.C. Molecular Shape and the Hydrophobic Effect. Annu. Rev. Phys. Chem. 2016, 67, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Papavasileiou, K.D.; Peristeras, L.D.; Bick, A.; Economou, I.G. Molecular Dynamics Simulation of Pure N-Alkanes and Their Mixtures at Elevated Temperatures Using Atomistic and Coarse-Grained Force Fields. J. Phys. Chem. B 2019, 123, 6229–6243. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, A.; Sharp, K.A.; Honig, B. Protein Folding and Association: Insights from the Interfacial and Thermodynamic Properties of Hydrocarbons. Proteins 1991, 11, 281–296. [Google Scholar] [CrossRef]
- Goodsaid-Zalduondo, F.; Engelman, D.M. Conformation of Liquid N-Alkanes. Biophys. J. 1981, 35, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Izvekov, S.; Voth, G.A. A Multiscale Coarse-Graining Method for Biomolecular Systems. J. Phys. Chem. B 2005, 109, 2469–2473. [Google Scholar] [CrossRef]
- Klauda, J.B.; Brooks, B.R.; MacKerell, A.D., Jr.; Venable, R.M.; Pastor, R.W. An Ab Initio Study on the Torsional Surface of Alkanes and Its Effect on Molecular Simulations of Alkanes and a DPPC Bilayer. J. Phys. Chem. B 2005, 109, 5300–5311. [Google Scholar] [CrossRef] [PubMed]
- Bochyński, Z.; Drozdowsκi, H. Structure and Molecular Correlation of Liquid Alkanes. Acta Phys. Pol. A 1995, 88, 1089–1096. [Google Scholar] [CrossRef]
- Habenschuss, A.; Johnson, E.; Narten, A.H. X-ray Diffraction Study and Models of Liquid Methane at 92 K. J. Chem. Phys. 1981, 74, 5234–5241. [Google Scholar] [CrossRef]
- Sandler, S.I.; Lombardo, M.G.; Wong, D.S.-H.; Habenschuss, A.; Narten, A.H. X-ray Diffraction Study and Models of Liquid Ethane at 105 and 181 K. J. Chem. Phys. 1982, 77, 2144–2152. [Google Scholar] [CrossRef]
- Habenschuss, A.; Narten, A.H. X-ray Diffraction Study of Liquid Propane at 92 and 228 K. J. Chem. Phys. 1986, 85, 6022–6026. [Google Scholar] [CrossRef]
- Habenschuss, A.; Narten, A.H. X-ray Diffraction Study of Liquid N-butane at 140 and 267 K. J. Chem. Phys. 1989, 91, 4299–4306. [Google Scholar] [CrossRef]
- Bochyński, Z.; Drozdowski, H. X-Ray Scattering in Liquid Pentane. Phys. Chem. Liq. 2001, 39, 189–199. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Luettmer-Strathmann, J.; Schoenhard, J.A.; Lipson, J.E.G. Thermodynamics of N-Alkane Mixtures and Polyethylene−n-Alkane Solutions: Comparison of Theory and Experiment. Macromolecules 1998, 31, 9231–9237. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An Ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Schuler, L.D.; Daura, X.; van Gunsteren, W.F. An Improved GROMOS96 Force Field for Aliphatic Hydrocarbons in the Condensed Phase. J. Comput. Chem. 2001, 22, 1205–1218. [Google Scholar] [CrossRef]
- Wang, J.; Hou, T. Application of Molecular Dynamics Simulations in Molecular Property Prediction I: Density and Heat of Vaporization. J. Chem. Theory Comput. 2011, 7, 2151–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, H.; Gao, W.; Su, L.; Sun, Z.; Chen, L. MD Simulation Study of the Diffusion and Local Structure of N-Alkanes in Liquid and Supercritical Methanol at Infinite Dilution. J. Mol. Model. 2017, 23, 195. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, G.; Sternfield, S.; Kumar, R.; Rick, S.W. Coarse-Grained Models of Aqueous and Pure Liquid Alkanes. J. Chem. Theory Comput. 2017, 13, 3846–3853. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.R.; Polik, W.F. WebMO Enterprise; WebMO LLC: Holland, MI, USA, 2020. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian16; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A Well-Behaved Electrostatic Potential Based Method Using Charge Restraints for Deriving Atomic Charges: The RESP Model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.; Brozell, S.R.; Cerutti, D.; Cheatham, T.; Cruzeiro, V.W.; Darden, T.; Duke, R.E.; Giambasu, G.; et al. Amber 2020; University of California: San Francisco, CA, USA, 2020. [Google Scholar]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.R., Jr. Thermochemical Data. In NIST Chemistry WebBook; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2021. [Google Scholar]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Tobler, F.C. Correlation of the Density of the Liquid Phase of Pure N-Alkanes with Temperature and Vapor Pressure. Ind. Eng. Chem. Res. 1998, 37, 2565–2570. [Google Scholar] [CrossRef]
- Caleman, C.; van Maaren, P.J.; Hong, M.; Hub, J.S.; Costa, L.T.; van der Spoel, D. Force Field Benchmark of Organic Liquids: Density, Enthalpy of Vaporization, Heat Capacities, Surface Tension, Isothermal Compressibility, Volumetric Expansion Coefficient, and Dielectric Constant. J. Chem. Theory Comput. 2012, 8, 61–74. [Google Scholar] [CrossRef]
- Tchouar, N.; Benyettou, M.; Kadour, F.O. Thermodynamic, Structural and Transport Properties of Lennard-Jones Liquid Systems. A Molecular Dynamics Simulations of Liquid Helium, Neon, Methane and Nitrogen. Int. J. Mol. Sci. 2003, 4, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Tchouar, N.; Benyettou, M.; Baghli, H. A Reversible Algorithm for Nosé Molecular Dynamics Simulations. Equilibrium Properties of Liquid Methane. J. Mol. Liq. 2007, 136, 5–10. [Google Scholar] [CrossRef]
- Petz, J.I. X-Ray Determination of the Structure of Liquid Methane. J. Chem. Phys. 1965, 43, 2238–2243. [Google Scholar] [CrossRef]
- Assael, M.J.; Dymond, J.H.; Exadaktilou, D. An Improved Representation Forn-Alkane Liquid Densities. Int. J. Thermophys. 1994, 15, 155–164. [Google Scholar] [CrossRef]
- Kraack, H.; Deutsch, M.; Sirota, E.B. N-Alkane Homogeneous Nucleation: Crossover to Polymer Behavior. Macromolecules 2000, 33, 6174–6184. [Google Scholar] [CrossRef]
- Lal, M.; Spencer, D. “Monte Carlo” Computer Simulation of Chain Molecules. Mol. Phys. 1973, 26, 1–6. [Google Scholar] [CrossRef]
- Romain, P.D.; Van, H.T.; Patterson, D. Effects of Molecular Flexibility and Shape on the Excess Enthalpies and Heat Capacities of Alkane Systems. J. Chem. Soc. Lond. Faraday Trans. 1 1979, 75, 1700–1707. [Google Scholar] [CrossRef]
- Sanjay, M.; Simanta, B.; Kulwant, S. Paraffin Problems in Crude Oil Production and Transportation: A Review. SPE Prod. Facil. 1995, 10, 50–54. [Google Scholar] [CrossRef]
- Anufriev, R.V.; Volkova, G.I.; Vasilyeva, A.A.; Petukhova, A.V.; Usheva, N.V. The Integrated Effect on Properties and Composition of High-Paraffin Oil Sludge. Procedia Chem. 2015, 15, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Kisliuk, A.; Sokolov, A.P. When Does a Molecule Become a Polymer? Macromolecules 2004, 37, 161–166. [Google Scholar] [CrossRef]
- Martin, M.G.; Siepmann, J.I. Transferable Potentials for Phase Equilibria. 1. United-Atom Description of N-Alkanes. J. Phys. Chem. B 1998, 102, 2569–2577. [Google Scholar] [CrossRef]
- Martin, M.G. Comparison of the AMBER, CHARMM, COMPASS, GROMOS, OPLS, TraPPE and UFF Force Fields for Prediction of Vapor–liquid Coexistence Curves and Liquid Densities. Fluid Phase Equilib. 2006, 248, 50–55. [Google Scholar] [CrossRef]



| Molecule | Boiling Point (K) a |
|---|---|
| Methane | 111. ± 2 |
| Ethane | 184.6 ± 0.6 |
| Propane | 231.1 ± 0.2 |
| Butane | 273. ± 1 |
| Pentane | 309.2 ± 0.2 |
| Hexane | 341.9 ± 0.3 |
| Heptane | 371.5 ± 0.3 |
| Octane | 398.7 ± 0.5 |
| Nonane | 423.8 ± 0.3 |
| Decane | 447.2 ± 0.3 |
| Undecane | 468. ± 2 |
| Dodecane | 489. ± 2 |
| Tridecane | 507. ± 2 |
| Tetradecane | 523. ± 10 |
| Pentadecane | 540. ± 20 |
| Molecule | Simulated | Experiment a | Percent Difference |
|---|---|---|---|
| Methane | 2.33 ± 0.02 | 2.06 | 13% |
| Ethane | 3.88 ± 0.05 | 3.75 | 3% |
| Propane | 5.25 ± 0.08 | 4.55 | 15% |
| Butane | 6.5 ± 0.1 | 5.47 | 20% |
| Pentane | 7.3 ± 0.1 | 6.33 | 16% |
| Hexane | 8.3 ± 0.2 | 7.41 | 13% |
| Heptane | 9.4 ± 0.2 | 8.60 | 10% |
| Octane | 10.2 ± 0.2 | 9.80 | 4% |
| Nonane | 11.1 ± 0.2 | 11.11 | 0.3% |
| Decane | 12.2 ± 0.3 | 12.26 | −0.4% |
| Undecane | 12.1 ± 0.3 | 13.48 | −10% |
| Dodecane | 13.1 ± 0.3 | 14.58 | −10% |
| Tridecane | 13.9 ± 0.3 | 15.94 | −13% |
| Tetradecane | 14.9 ± 0.4 | 17.11 | −13% |
| Pentadecane | 15.6 ± 0.4 | 18.19 | −14% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindberg, G.E.; Baker, J.L.; Hanley, J.; Grundy, W.M.; King, C. Density, Enthalpy of Vaporization and Local Structure of Neat N-Alkane Liquids. Liquids 2021, 1, 47-59. https://0-doi-org.brum.beds.ac.uk/10.3390/liquids1010004
Lindberg GE, Baker JL, Hanley J, Grundy WM, King C. Density, Enthalpy of Vaporization and Local Structure of Neat N-Alkane Liquids. Liquids. 2021; 1(1):47-59. https://0-doi-org.brum.beds.ac.uk/10.3390/liquids1010004
Chicago/Turabian StyleLindberg, Gerrick E., Joseph L. Baker, Jennifer Hanley, William M. Grundy, and Caitlin King. 2021. "Density, Enthalpy of Vaporization and Local Structure of Neat N-Alkane Liquids" Liquids 1, no. 1: 47-59. https://0-doi-org.brum.beds.ac.uk/10.3390/liquids1010004






