Animal Models in Neuroscience: What Is the “Culture of Care”?
Abstract
:1. Introduction
1.1. Ethical Considerations: Prioritizing Animal Welfare and Scientific Progress
1.2. Ethical Paradigms: Anthropocentrism and Pathocentrism Examined
1.3. Synergizing Ethical Compasses: Comparing the Five Freedoms and the Five Domains
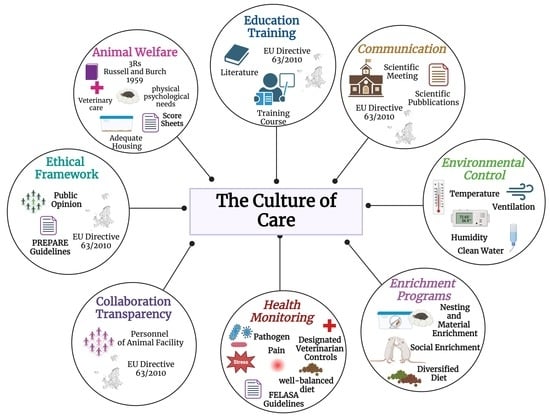
2. The Culture of Care
2.1. Embracing Ethical Excellence: Cultivating a “Culture of Care” in Animal Research
- Animal welfare: Animal welfare precedes research objectives or convenience. It involves the provision of adequate housing, veterinary care, and attention to the physical and psychological needs of animals [19,45,62]. Knowledge about AMs to be acquired includes information about the species and the different strains, focusing on transgenic ones [19,45,62]. Adopting score sheets can help identify these signs of distress early, facilitating prompt intervention [63,64,65]. Several models are available in the bibliography (e.g., [25,66,67,68]). It is essential to customize models to fit specific needs based on the type of research and animal model being used.
- Ethical framework: The culture of care should be actively implemented daily, not just as an abstract concept [16,35,45]. Establishing a robust ethical framework involves defining clear guidelines and policies that promote respect, kindness, and ethical treatment of animals [7,39,42]. This includes exceeding minimal legal requirements and ensuring compliance with ethical standards in all research activities [7,39,42].
- Education and training: To ensure proper animal care, all personnel must receive adequate training as described in the referred normative (European Directive 2010/63/EU) [69]. This helps maintain high morale, skill development, and compliance with best practices in animal welfare [27,49,69]. All personnel working with laboratory animals must acquire the information and updates essential to embracing the culture of care carefully, including those who perform procedures on animals (Function A), those who design the experimental study (Function B), those who take care of the animals (Function C), and those who euthanize the animals (Function D) [27,49,69]. Everyone needs to be well versed in current regulations; the specific animal model they are working with; and all strategies to ensure animal welfare, starting with the 3Rs principle [16,17]. A comprehensive knowledge of the animal model, encompassing its characteristics and limitations, and careful analysis of the relevant literature can guide efforts towards replacement and reduction [17]. This knowledge empowers people to consider an NAM that is more suitable and informative for all or part of the study [27,49,69].
- Collaboration and transparency: In AM studies, transparency concerns the accurate sharing of the results and the chosen experimental methods, including the selection, care, and use of laboratory animals [70,71,72]. Detailed information on experiment design, ethical procedures adopted, and animal welfare monitoring should be provided [70,71,72]. Transparency and effective communication also include disclosing any limitations or challenges encountered during the research study [70,71,72]. This honest approach fosters a deeper comprehension of the studies conducted and facilitates mutual learning among researchers [70,71,72]. Collaboration and transparency play pivotal roles in establishing a robust knowledge base in neuroscience, ensuring that research is ethically grounded and that results are beneficial for the progress of science and medicine. Clarity in communication is equally important, not only among researchers [70,71,72].
- Health monitoring and environmental control: Monitoring the health of animal colonies is crucial to obtaining reliable scientific data [31]. It helps prevent variables in experimental designs and safeguards personnel health [31,69,73]. All animal facilities must have a periodic health monitoring program for pathogens [69,73]. Health controls can be either direct or indirect: tests on the animals themselves, their products, the environment in which they live, and the personnel involved in their management [69]. Effective research requires proper planning with established timelines and a clear list of pathogens. The Federation of European Laboratory Animal Science Associations (FELASA) guidelines and standardized health reports aid information exchange among cooperating labs [56,69,73]. Daily observations made by staff are crucial, in addition to routine health surveillance, assays, sampling, and testing [69,74,75]. Environmental control is also essential to ensuring animal health. Monitoring environmental parameters such as temperature, humidity, and ventilation carefully and regularly helps prevent the spread of diseases [75].
- Enrichment programs: Environmental and social enrichment are crucial to ensuring the welfare of laboratory animals [81,82,83]. Programs should be customized based on the specific needs and behaviors of the species involved while complying with applicable regulations and ethical principles. Providing adequate space and complexity is essential to allowing animals to express normal species-specific behaviors [81,82,83,84].
- Communication: To have a successful team, it is essential to have individuals inclined towards communication and collaboration while having clearly defined roles [59,86,87]. This is essential to ensuring animal welfare and generate reliable scientific results that can be easily reproduced and translated to humans [27,87].
2.2. Challenges and Strategies in Implementing a Culture of Care in Animal Facilities
3. Bridging Animal Models with a Culture of Care in Neuroscience
3.1. Comprehensive Perspectives in Neuroscience Research: Animal Models, Advances, and Ethical Considerations
3.2. Advancing Neuroscience Responsibly: Exploring Alternative Methods within a Culture of Care
- -
- Computer models: Computer models in neuroscience serve as a transformative tool for researchers and practitioners, providing a comprehensive platform for simulating intricate neural processes [132,133]. This simulation enables a deep exploration of the behaviors of neurons, synaptic connections, and neural networks, shedding light on how the brain processes information and generates complex behaviors. Beyond fundamental neuroscience, these computational models play a pivotal role in drug discovery and development, with applications like computer-aided drug design (CADD) predicting drug–receptor interactions and expediting the identification of therapeutic compounds [134]. Moreover, these models contribute significantly to understanding the underlying mechanisms of various neurological and psychiatric disorders, offering insights into conditions such as epilepsy, Alzheimer’s disease, and schizophrenia [135,136]. In diagnostics, advanced computational techniques analyze neuroimaging data, employing machine learning algorithms to identify patterns in brain scans and enhance diagnostic and prognostic capabilities [135,136]. Additionally, computer models are integral to developing brain–machine interfaces, fostering communication between the brain and external devices, with potential applications in assisting individuals with paralysis [135,136]. These models also contribute to cognitive modeling, helping unravel the intricacies of how the brain processes information, learns, and makes decisions. In personalized medicine, computational models analyze individual genetic, neuroimaging, and clinical data to predict responses to specific treatments, paving the way for more tailored and effective therapeutic interventions [135,136]. Furthermore, these models serve educational initiatives by providing interactive and visual tools for learning, allowing students to explore complex concepts and enhance their understanding of neural processes. In summary, computer models in neuroscience represent a versatile and powerful toolbox, contributing to advancements in drug development, the understanding of brain function and disorders, and the improvement in diagnostic and therapeutic strategies [135,136].
- -
- Cells and tissue cultures are vital components in neuroscience research, offering versatile platforms for delving into the intricacies of the NS [137,138,139]. These in vitro models serve many purposes, from studying fundamental aspects of neuronal function and communication to modeling neurological disorders. In the context of drug screening and development, these cultures provide a controlled environment to assess the effects of potential therapeutic compounds on neuronal cells [137,138,139]. Additionally, they play a pivotal role in toxicology studies, allowing researchers to evaluate the impact of various substances on neuronal health without resorting to animal experimentation [137,138,139]. The application of these cultures extends to electrophysiological studies, offering insights into the electrical activity of neurons and their networks [137,138,139]. Furthermore, neural stem cell-derived cultures contribute to exploring neuroregeneration and repair mechanisms, providing valuable information for developing strategies to promote neural recovery [137,138,139]. These in vitro models also play a crucial role in investigating neurodevelopment, gene expression, and other facets that collectively enhance our understanding of the complex workings of the NS [137,138,139].
- -
- Organoids, miniature three-dimensional tissue structures cultivated in vitro, represent a revolutionary tool in neuroscience research [140,141,142]. These self-organizing structures, resembling simplified organs, offer a unique opportunity to study complex aspects of brain development and function in a controlled environment [140,141,142]. In neuroscience, organoids are employed to model various aspects of the brain, allowing researchers to explore neuronal connectivity, synapse formation, and the development of specific brain regions [140,141,142]. Furthermore, organoids derived from patient cells enable the modeling of neurological disorders, providing insights into disease mechanisms and potential therapeutic interventions [140,141,142]. Their application extends to drug testing, where organoids serve as a valuable platform for screening and evaluating the efficacy of pharmaceutical compounds [140,141,142]. The ability to reproduce critical features of the brain’s architecture and functionality makes organoids a powerful tool for advancing our understanding of neurobiology and addressing intricate questions related to brain development, diseases, and potential treatment strategies [140,141,142].
| NAMs | Implication in Neuroscience | Ref. |
|---|---|---|
| fMRI and PET | Detailed and controlled investigations into cellular and molecular mechanisms without ethical concerns about animal use. | [27,30,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,131] |
| Computer models | Transformative tool for researchers and practitioners providing a comprehensive platform for simulating intricate neuronal processes allowing for the following:
| [133,134,135,136] |
| Cells and tissue cultures | Used for studying fundamental aspects of neuronal function and communication to model neurological disorders, allowing for the following:
| [137,138,139] |
| Organoids | Resembling simplified organs, they offer a unique opportunity to study complex aspects of brain development and function in a controlled environment, allowing for the following:
| [140,141,142] |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMs | animal models |
| NS | nervous system |
| NAMs | new alternative models |
| AWBs | animal welfare bodies |
| 3Rs | replacement, reduction, and refinement |
| SPF | specific pathogen-free |
| LAA | laboratory animal allergies |
| GF | germ-free |
| FELASA | Federation of European Laboratory Animal Science Associations |
| GLAPs | good laboratory animal practices |
| EE | environmental enrichment |
References
- Lambert, K. Wild brains: The value of neuroethological approaches in preclinical behavioral neuroscience animal models. Neurosci. Biobehav. Rev. 2023, 146, 105044. [Google Scholar] [CrossRef]
- Romanova, E.V.; Sweedler, J.V. Animal model systems in neuroscience. ACS Chem. Neurosci. 2018, 9, 1869–1870. [Google Scholar] [CrossRef]
- Bovenkerk, B.; Kaldewaij, F. The use of animal models in behavioural neuroscience research. Curr. Top. Behav. Neurosci. 2015, 19, 17–46. [Google Scholar] [CrossRef]
- Crystal, J.D. Elements of episodic-like memory in animal models. Behav. Process. 2009, 80, 269–277. [Google Scholar] [CrossRef]
- Yanshree; Yu, W.S.; Fung, M.L.; Lee, C.W.; Lim, L.W.; Wong, K.H. The monkey head mushroom and memory enhancement in Alzheimer’s disease. Cells 2022, 11, 2284. [Google Scholar] [CrossRef]
- Fine, A.H.; Beck, A.M.; Ng, Z. The State of Animal-Assisted Interventions: Addressing the Contemporary Issues that will Shape the Future. Int. J. Environ. Res. Public Health 2019, 16, 3997. [Google Scholar] [CrossRef]
- DeGrazia, D.; Beauchamp, T.L. Beyond the 3Rs to a more comprehensive framework of principles for animal research ethics. ILAR J. 2021, 60, 308–317. [Google Scholar] [CrossRef]
- Gruen, L. Ethics and Animals: An Introduction; Cambridge University Press: Cambridge, UK, 2021; ISBN 9781108988544. [Google Scholar]
- Robinson, S.; Sparrow, S.; Williams, B.; Decelle, T.; Bertelsen, T.; Reid, K.; Chlebus, M. The European Federation of the Pharmaceutical Industry and Associations’ Research and Animal Welfare Group: Assessing and benchmarking “Culture of Care” in the context of using animals for scientific purpose. Lab. Anim. 2019, 54, 23677219887998. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.S.; Goerlich, V.C.; van der Staay, F.J. A dynamic concept of animal welfare: The role of appetitive and adverse internal and external factors and the animal’s ability to adapt to them. Front. Anim. Sci. 2022, 3. [Google Scholar] [CrossRef]
- Davies, G.; Gorman, R.; Greenhough, B.; Hobson-West, P.; Kirk, R.G.W.; Message, R.; Myelnikov, D.; Palmer, A.; Roe, E.; Ashall, V.; et al. Animal research nexus: A new approach to the connections between science, health and animal welfare. Med. Humanit. 2020, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Regan, T. Animal Rights, Human Wrongs: An Introduction to Moral Philosophy; Rowman & Littlefield Publishers: Lanham, MD, USA, 2003; ISBN 9780742599383. [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011; ISBN 0309154006. [Google Scholar]
- Ferrara, F.; Hiebl, B.; Kunzmann, P.; Hutter, F.; Afkham, F.; LaFollette, M.; Gruber, C. Culture of care in animal research—Expanding the 3Rs to include people. Lab. Anim. 2022, 56, 511–518. [Google Scholar] [CrossRef]
- Soulsbury, C.; Gray, H.; Smith, L.; Braithwaite, V.; Cotter, S.; Elwood, R.W.; Wilkinson, A.; Collins, L.M. The welfare and ethics of research involving wild animals: A primer. Methods Ecol. Evol. 2020, 11, 1164–1181. [Google Scholar] [CrossRef]
- Wahyuwardani, S.; Noor, S.M.; Bakrie, B. Animal welfare ethics in research and testing: Implementation and its barrier. WARTAZOA 2020, 30, 211. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, D.W.; Kang, B.C. The “R” principles in laboratory animal experiments. Lab. Anim. Res. 2020, 36, 45. [Google Scholar] [CrossRef]
- Blumer, K. Ethical aspects of animal experiments and the principle of solidarity. In Deutsche Forschungsgemeinschaft (DFG). Animal Experiments in Research; Exner, C., Bode, H.-J., Blumer, C., Giese, C., Eds.; Lemmens Medien: Bonn, Germany, 2007. [Google Scholar]
- Martinez, J.; von Nolting, C. Review: “Animal welfare”—A European concept. Animal 2023, 17 (Suppl. 4), 100839. [Google Scholar] [CrossRef]
- Maple, T.L.; Bloomsmith, M.A. Introduction: The science and practice of optimal animal welfare. Behav. Process. 2018, 156, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef] [PubMed]
- National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research. Guidance for the Description of Animal Research in Scientific Publications; National Academies Press: Washington, DC, USA, 2011; ISBN 9780309219518. [Google Scholar]
- Garner, J.P. The significance of meaning: Why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? ILAR J. 2014, 55, 438–456. [Google Scholar] [CrossRef] [PubMed]
- Coscas, R.; Senemaud, J. Experimenters or Amateurs? Eur. J. Vasc. Endovasc. Surg. 2020, 60, 253. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, J.; Asselbergs, F.W.; Bakkers, J.; Batkai, S.; Bertrand, L.; Bezzina, C.R.; Bot, I.; Brundel, B.J.J.M.; Carrier, L.; Chamuleau, S.; et al. Animal models and animal-free innovations for cardiovascular research: Current status and routes to be explored. Consensus document of the ESC Working Group on Myocardial Function and the ESC Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2022, 118, 3016–3051. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, D.M.; Feeney, W.P. The influence of feed and drinking water on terrestrial animal research and study replicability. ILAR J. 2020, 60, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Symonowicz, C.; Medina, L.V.; Bratcher, N.A.; Buckmaster, C.A.; Klein, H.; Anderson, L.C. Culture of care: Organizational responsibilities. In Management of Animal Care and Use Programs in Research, Education, and Testing; Weichbrod, R.H., Thompson, G.A., Norton, J.N., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; ISBN 9781315152189. [Google Scholar]
- Bertelsen, T.; Øvlisen, K. Assessment of the Culture of Care working with laboratory animals by using a comprehensive survey tool. Lab. Anim. 2021, 55, 453–462. [Google Scholar] [CrossRef]
- Williams, A. Caring for those who care: Towards a more expansive understanding of ‘cultures of care’ in laboratory animal facilities. Soc. Cult. Geogr. 2023, 24, 31–48. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and humane experimental technique: Implementing change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Buchheister, S.; Bleich, A. Health Monitoring of Laboratory Rodent Colonies-Talking about (R)evolution. Animals 2021, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Landscape sustainability science: Ecosystem services and human well-being in changing landscapes. Landsc. Ecol. 2013, 28, 999–1023. [Google Scholar] [CrossRef]
- Liz Paola, N.Z.; Torgerson, P.R.; Hartnack, S. Alternative paradigms in animal health decisions: A framework for treating animals not only as commodities. Animals 2022, 12, 1845. [Google Scholar] [CrossRef]
- Kopnina, H.; Washington, H.; Taylor, B.; Piccolo, J.J. Anthropocentrism: More than Just a Misunderstood Problem. J. Agric. Environ. Ethics 2018, 31, 109–127. [Google Scholar] [CrossRef]
- Croney, C.C.; Anthony, R. Engaging science in a climate of values: Tools for animal scientists tasked with addressing ethical problems. J. Anim. Sci. 2010, 88, E75–E81. [Google Scholar] [CrossRef]
- Beausoleil, N.J. I am a compassionate conservation welfare scientist: Considering the theoretical and practical differences between compassionate conservation and conservation welfare. Animals 2020, 10, 257. [Google Scholar] [CrossRef]
- Eggel, M.; Camenzind, S. Authorization of animal research proposals—A comparison of harm concepts in different European regulations. Berl. Münchener Tierärztliche Wochenschr. 2020. online first. [Google Scholar] [CrossRef]
- Baertschi, B.; Gyger, M. Ethical considerations in mouse experiments. Curr. Protoc. Mouse Biol. 2011, 1, 155–167. [Google Scholar] [CrossRef]
- Gross, D.; Tolba, R.H. Ethics in Animal-Based Research. Eur. Surg. Res. 2015, 55, 43–57. [Google Scholar] [CrossRef]
- Grimm, H. Ethics in laboratory animal science. In Comparative Medicine; Jensen-Jarolim, E., Ed.; Springer: Vienna, Austria, 2014; pp. 281–300. ISBN 978-3-7091-1558-9. [Google Scholar]
- Millar, K.M. Translational stem cell research and animal use: Examining ethical issues and opportunities. In Translational Stem Cell Research; Hug, K., Hermerén, G., Eds.; Stem Cell Biology and Regenerative Medicine; Humana Press: Totowa, NJ, USA, 2011; pp. 113–124. ISBN 978-1-60761-958-1. [Google Scholar]
- Vorstenbosch, J.M.G. The ethics of the Three Rs principle: A reconsideration. Anim. Welf. 2005, 14, 339–345. [Google Scholar] [CrossRef]
- Grunwald, A. Living Technology: Philosophy and Ethics at the Crossroads between Life and Technology; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781000346428. [Google Scholar]
- Schindler, S. The animal’s dignity in Swiss Animal Welfare Legislation—Challenges and opportunities. Eur. J. Pharm. Biopharm. 2013, 84, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K. Concepts of animal welfare in relation to positions in animal ethics. Acta Biotheor. 2011, 59, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Mannhold, R.; Kubinyi, H.; Folkers, G. Animal Models for Human Cancer: Discovery and Development of Novel Therapeutics; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9783527339976. [Google Scholar]
- Arlinghaus, R.; Cooke, S.J.; Lyman, J.; Policansky, D.; Schwab, A.; Suski, C.; Sutton, S.G.; Thorstad, E.B. Understanding the Complexity of Catch-and-Release in Recreational Fishing: An Integrative Synthesis of Global Knowledge from Historical, Ethical, Social, and Biological Perspectives. Rev. Fish. Sci. 2007, 15, 75–167. [Google Scholar] [CrossRef]
- McCausland, C. The five freedoms of animal welfare are rights. J. Agric. Environ. Ethics 2014, 27, 649–662. [Google Scholar] [CrossRef]
- Mellor, D.J. Moving beyond the “Five Freedoms” by Updating the “Five Provisions” and Introducing Aligned “Animal Welfare Aims”. Animals 2016, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Serpell, J.A.; Coppinger, R.; Fine, A.H.; Peralta, J.M. Welfare considerations in therapy and assistance animals. In Handbook on Animal-Assisted Therapy; Elsevier: Amsterdam, The Netherlands, 2010; pp. 481–503. ISBN 9780123814531. [Google Scholar]
- Jaasma, L. A Review of the Housing Conditions for Laboratory Animals. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, July 2014. [Google Scholar]
- Gregory, N.G. Physiology and Behaviour of Animal Suffering; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9781405173025. [Google Scholar]
- Mellor, D.J. Operational details of the five domains model and its key applications to the assessment and management of animal welfare. Animals 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human-Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J. Extending the ‘Five Domains’ model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- Gyger, M.; Berdoy, M.; Dontas, I.; Kolf-Clauw, M.; Santos, A.I.; Sjöquist, M. FELASA accreditation of education and training courses in laboratory animal science according to the Directive 2010/63/EU. Lab. Anim. 2019, 53, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Azkona, G.; Sanchez-Pernaute, R. Mice in translational neuroscience: What R we doing? Prog. Neurobiol. 2022, 217, 102330. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.; Hartley, S. Responsibility and laboratory animal research governance. Sci. Technol. Hum. Values 2018, 43, 723–741. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.F.; Greenhough, B.J.; Hobson-West, P.; Kirk, R.G.W.; Applebee, K.; Bellingan, L.C.; Berdoy, M.; Buller, H.; Cassaday, H.J.; Davies, K.; et al. Developing a collaborative agenda for humanities and social scientific research on laboratory animal science and welfare. PLoS ONE 2016, 11, e0158791. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Greenhough, B.; Hobson-West, P.; Kirk, R.G.W. Science, culture, and care in laboratory animal research. Sci. Technol. Hum. Values 2018, 43, 016224391875703. [Google Scholar] [CrossRef]
- Rolland, B.; Burnside, E.S.; Voils, C.I.; Shah, M.N.; Brasier, A.R. Enhancing reproducibility using interprofessional team best practices. J. Clin. Transl. Sci. 2020, 5, e20. [Google Scholar] [CrossRef]
- Mellor, D.J. Updating Animal Welfare Thinking: Moving beyond the “Five Freedoms” towards “A Life Worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef]
- Planchez, B.; Surget, A.; Belzung, C. Animal models of major depression: Drawbacks and challenges. J. Neural Transm. 2019, 126, 1383–1408. [Google Scholar] [CrossRef]
- Leenaars, M.; Hooijmans, C.R.; van Veggel, N.; ter Riet, G.; Leeflang, M.; Hooft, L.; van der Wilt, G.J.; Tillema, A.; Ritskes-Hoitinga, M. A step-by-step guide to systematically identify all relevant animal studies. Lab. Anim. 2012, 46, 24–31. [Google Scholar] [CrossRef]
- Hawkins, P. Recognizing and assessing pain, suffering and distress in laboratory animals: A survey of current practice in the UK with recommendations. Lab. Anim. 2002, 36, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, A.C.; Bendotti, C.; Blaugrund, E.; Chio, A.; Greensmith, L.; Loeffler, J.-P.; Mead, R.; Niessen, H.G.; Petri, S.; Pradat, P.-F.; et al. Guidelines for preclinical animal research in ALS/MND: A consensus meeting. Amyotroph. Lateral Scler. 2010, 11, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef] [PubMed]
- Zemanova, M.A. Crucial but neglected: Limited availability of animal welfare courses in education of wildlife researchers. Animals 2023, 13, 2907. [Google Scholar] [CrossRef] [PubMed]
- Scavizzi, F.; Galligioni, V.; Vasina, V.; Raspa, M. Animal health management and hygiene. In Practical Handbook on the 3Rs in the Context of the Directive 2010/63/EU.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 151–179. ISBN 9780128211809. [Google Scholar]
- Hooijmans, C.R.; de Vries, R.B.M.; Ritskes-Hoitinga, M.; Rovers, M.M.; Leeflang, M.M.; IntHout, J.; Wever, K.E.; Hooft, L.; de Beer, H.; Kuijpers, T.; et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS ONE 2018, 13, e0187271. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Carbone, L.; Austin, J. Pain and laboratory animals: Publication practices for better data reproducibility and better animal welfare. PLoS ONE 2016, 11, e0155001. [Google Scholar] [CrossRef]
- Nicklas, W. International harmonization of health monitoring. ILAR J. 2008, 49, 338–346. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals Environment, Housing, and Management. 2011. Available online: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf (accessed on 20 November 2023).
- National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2010; ISBN 9780309186636. [Google Scholar]
- Temple, D.; Manteca, X. Animal welfare in extensive production systems is still an area of concern. Front. Sustain. Food Syst. 2020, 4, 545902. [Google Scholar] [CrossRef]
- Martin, A.L.; Franklin, A.N.; Perlman, J.E.; Bloomsmith, M.A. Systematic assessment of food item preference and reinforcer effectiveness: Enhancements in training laboratory-housed rhesus macaques. Behav. Process. 2018, 157, 445–452. [Google Scholar] [CrossRef]
- Germ-Free Animals: A Key Tool in Unraveling How the Microbiota Affects the Brain and Behavior. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/abs/pii/B9780323999717000126 (accessed on 29 December 2023).
- Luczynski, P.; McVey Neufeld, K.-A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016, 19, pyw020. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Weiper, K.; Tolba, R.H.; Weiskirchen, R. All You Can Feed: Some Comments on Production of Mouse Diets Used in Biomedical Research with Special Emphasis on Non-Alcoholic Fatty Liver Disease Research. Nutrients 2020, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, L.; Zhang, X. Environmental enrichment increases aquatic animal welfare: A systematic review and meta-analysis. Rev. Aquacult. 2022, 14, 1120–1135. [Google Scholar] [CrossRef]
- Lee, G.-H.; Kim, K.; Jo, W. Stress evaluation of mouse husbandry environments for improving laboratory animal welfare. Animals 2023, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Baumans, V.; Van Loo, P.L.P. How to improve housing conditions of laboratory animals: The possibilities of environmental refinement. Vet. J. 2013, 195, 24–32. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, C.S.; Cipreste, C.F.; Pizzutto, C.S.; Young, R.J. Review of the effects of enclosure complexity and design on the behaviour and physiology of zoo animals. Animals 2023, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Baumans, V. Environmental enrichment for laboratory rodents and rabbits: Requirements of rodents, rabbits, and research. ILAR J. 2005, 46, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; White, W.; Wilkes, J.; Wilkinson, C. Improving culture of care through maximising learning from observations and events: Addressing what is at fault. Lab. Anim. 2022, 56, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Bayne, K.A. Establishing a culture of care, conscience, and responsibility: Addressing the improvement of scientific discovery and animal welfare through science-based performance standards. ILAR J. 2007, 48, 3–11. [Google Scholar] [CrossRef]
- Bekoff, M.; Meaney, C.A. (Eds.) Encyclopedia of Animal Rights and Animal Welfare; Routledge: Oxfordshire, UK, 2013; ISBN 9781135930028. [Google Scholar]
- Chow, P.K.H.; Ng, T.H.R.; Ogden, B.E. Using Animal Models in Biomedical Research: A Primer for the Investigator; World Scientific: Singapore, 2008; ISBN 9789812812025. [Google Scholar]
- Van Norman, G.A. Limitations of animal studies for predicting toxicity in clinical trials: Part 2: Potential alternatives to the use of animals in preclinical trials. JACC Basic Transl. Sci. 2020, 5, 387–397. [Google Scholar] [CrossRef]
- Mushtaq, S.; Daş, Y.K.; Aksoy, A. Alternative methods to animal experiments. Turkiye Klinikleri J. Lab. Anim. 2018, 170, 68–76. [Google Scholar] [CrossRef]
- Scott, J.T.; Bourne, J.A. Modelling behaviors relevant to brain disorders in the nonhuman primate: Are we there yet? Prog. Neurobiol. 2022, 208, 102183. [Google Scholar] [CrossRef]
- Hajar, R. Animal testing and medicine. Heart Views 2011, 12, 42. [Google Scholar] [CrossRef]
- Distelzweig, P. “Mechanics” and mechanism in william harvey’s anatomy: Varieties and limits. In Early Modern Medicine and Natural Philosophy; Distelzweig, P., Goldberg, B., Ragland, E.R., Eds.; History, philosophy and theory of the life sciences; Springer: Dordrecht, The Netherlands, 2016; Volume 14, pp. 117–140. ISBN 978-94-017-7352-2. [Google Scholar]
- van Helvoort, T. Bacteriological and physiological research styles in the early controversy on the nature of the bacteriophage phenomenon. Med. Hist. 1992, 36, 243–270. [Google Scholar] [CrossRef]
- Wang, N.; Anderson, R.J.; Ashbrook, D.G.; Gopalakrishnan, V.; Park, Y.; Priebe, C.E.; Qi, Y.; Laoprasert, R.; Vogelstein, J.T.; Williams, R.W.; et al. Variability and heritability of mouse brain structure: Microscopic MRI atlases and connectomes for diverse strains. Neuroimage 2020, 222, 117274. [Google Scholar] [CrossRef]
- Mitchell, A.S.; Hartig, R.; Basso, M.A.; Jarrett, W.; Kastner, S.; Poirier, C. International primate neuroscience research regulation, public engagement and transparency opportunities. Neuroimage 2021, 229, 117700. [Google Scholar] [CrossRef]
- Muley, M.M.; Krustev, E.; McDougall, J.J. Preclinical assessment of inflammatory pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. The human connectome: A complex network. Ann. N. Y. Acad. Sci. 2011, 1224, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Ohl, F.; Meijboom, F. Ethical issues associated with the use of animal experimentation in behavioral neuroscience research. Curr. Top. Behav. Neurosci. 2015, 19, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Vieira de Castro, A.C.; Olsson, I.A.S. Does the goal justify the methods? Harm and benefit in neuroscience research using animals. Curr. Top. Behav. Neurosci. 2015, 19, 47–78. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Yunta, E. Ethical issues concerning genetically modified animals for the study of human diseases. In Handbook of Bioethical Decisions. Volume I: Decisions at the Bench; Valdés, E., Lecaros, J.A., Eds.; Collaborative Bioethics; Springer International Publishing: Cham, Switzerland, 2023; Volume 2, pp. 513–525. ISBN 978-3-031-29450-1. [Google Scholar]
- Tello, J.A.; Williams, H.E.; Eppler, R.M.; Steinhilb, M.L.; Khanna, M. Animal models of neurodegenerative disease: Recent advances in fly highlight innovative approaches to drug discovery. Front. Mol. Neurosci. 2022, 15, 883358. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.S.; Horien, C.; Barson, D.; Scheinost, D.; Constable, R.T. Why is everyone talking about brain state? Trends Neurosci. 2023, 46, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Thurm, A.; Ethridge, S.B.; Soller, M.M.; Petkova, S.P.; Abel, T.; Bauman, M.D.; Brodkin, E.S.; Harony-Nicolas, H.; Wöhr, M.; et al. Reconsidering animal models used to study autism spectrum disorder: Current state and optimizing future. Genes Brain Behav. 2022, 21, e12803. [Google Scholar] [CrossRef] [PubMed]
- Moon, C. New Insights into and Emerging Roles of Animal Models for Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 4957. [Google Scholar] [CrossRef] [PubMed]
- Eichmüller, O.L.; Knoblich, J.A. Human cerebral organoids—A new tool for clinical neurology research. Nat. Rev. Neurol. 2022, 18, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Panoutsopoulos, A.A. Organoids, Assembloids, and Novel Biotechnology: Steps Forward in Developmental and Disease-Related Neuroscience. Neuroscientist 2021, 27, 463–472. [Google Scholar] [CrossRef]
- Wilcox, R.R.; Rousselet, G.A. A guide to robust statistical methods in neuroscience. Curr. Protoc. Neurosci. 2018, 82, 8.42.1–8.42.30. [Google Scholar] [CrossRef]
- Jucker, M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat. Med. 2010, 16, 1210–1214. [Google Scholar] [CrossRef]
- Feng, G.; Jensen, F.E.; Greely, H.T.; Okano, H.; Treue, S.; Roberts, A.C.; Fox, J.G.; Caddick, S.; Poo, M.-M.; Newsome, W.T.; et al. Opportunities and limitations of genetically modified nonhuman primate models for neuroscience research. Proc. Natl. Acad. Sci. USA 2020, 117, 24022–24031. [Google Scholar] [CrossRef]
- Kafkafi, N.; Agassi, J.; Chesler, E.J.; Crabbe, J.C.; Crusio, W.E.; Eilam, D.; Gerlai, R.; Golani, I.; Gomez-Marin, A.; Heller, R.; et al. Reproducibility and replicability of rodent phenotyping in preclinical studies. Neurosci. Biobehav. Rev. 2018, 87, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.D.; Shaevitz, J.W.; Murthy, M. Quantifying behavior to understand the brain. Nat. Neurosci. 2020, 23, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Brenowitz, E.A.; Zakon, H.H. Emerging from the bottleneck: Benefits of the comparative approach to modern neuroscience. Trends Neurosci. 2015, 38, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Forstmann, B.U.; Ratcliff, R.; Wagenmakers, E.J. Sequential sampling models in cognitive neuroscience: Advantages, applications, and extensions. Annu. Rev. Psychol. 2016, 67, 641–666. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.C.; Smith, M.A. Animal models of resistance exercise and their application to neuroscience research. J. Neurosci. Methods 2016, 273, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Sécher, T.; Bodier-Montagutelli, E.; Guillon, A.; Heuzé-Vourc’h, N. Correlation and clinical relevance of animal models for inhaled pharmaceuticals and biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 167, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Boelsterli, U.A. Healthy animals and animal models of human disease(s) in safety assessment of human pharmaceuticals, including therapeutic antibodies. Drug Discov. Today 2007, 12, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Lee-Liu, D.; Méndez-Olivos, E.E.; Muñoz, R.; Larraín, J. The African clawed frog Xenopus laevis: A model organism to study regeneration of the central nervous system. Neurosci. Lett. 2017, 652, 82–93. [Google Scholar] [CrossRef]
- Chalfie, M.; Jorgensen, E.M. C. elegans neuroscience: Genetics to genome. Trends Genet. 1998, 14, 506–512. [Google Scholar] [CrossRef]
- Sengupta, P.; Samuel, A.D.T. Caenorhabditis elegans: A model system for systems neuroscience. Curr. Opin. Neurobiol. 2009, 19, 637–643. [Google Scholar] [CrossRef]
- Husson, S.J.; Gottschalk, A.; Leifer, A.M. Optogenetic manipulation of neural activity in C. elegans: From synapse to circuits and behaviour. Biol. Cell 2013, 105, 235–250. [Google Scholar] [CrossRef]
- Gerlai, R. Zebrafish (Danio rerio): A newcomer with great promise in behavioral neuroscience. Neurosci. Biobehav. Rev. 2023, 144, 104978. [Google Scholar] [CrossRef]
- Davis, R.L. Learning and memory using Drosophila melanogaster: A focus on advances made in the fifth decade of research. Genetics 2023, 224, iyad085. [Google Scholar] [CrossRef] [PubMed]
- Liguori, F.; Pandey, U.B.; Digilio, F.A. Editorial: Drosophila as a model to study neurodegenerative diseases. Front. Neurosci. 2023, 17, 1275253. [Google Scholar] [CrossRef] [PubMed]
- Rinkwitz, S.; Mourrain, P.; Becker, T.S. Zebrafish: An integrative system for neurogenomics and neurosciences. Prog. Neurobiol. 2011, 93, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Model. Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N. Model homes for model organisms: Intersections of animal welfare and behavioral neuroscience around the environment of the laboratory mouse. Biosocieties 2016, 11, 46–66. [Google Scholar] [CrossRef]
- Prescott, M.J.; Lidster, K. Improving quality of science through better animal welfare: The NC3Rs strategy. Lab. Anim. 2017, 46, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.T.; Far, Y.K.; Ghaemi, Z.; Kamran, Z.; Andalibian, M.; Farhanian, A.; Taleifard, A.; Sanaei, A.; Chaboki, F.; Heidary, S. Neuroscience and Technology: Innovations in Brain Research and Therapy; Nobel Sciences: Dighi, India, 2023. [Google Scholar]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef]
- Dewhurst, D.G.; Hardcastle, J.; Hardcastle, P.T.; Stuart, E. Comparison of a computer simulation program and a traditional laboratory practical class for teaching the principles of intestinal absorption. Am. J. Physiol. 1994, 267, S95–S104. [Google Scholar] [CrossRef]
- Knight, A.; Bailey, J.; Balcombe, J. Animal carcinogenicity studies: 3. Alternatives to the bioassay. Altern. Lab. Anim. 2006, 34, 39–48. [Google Scholar] [CrossRef]
- Levenstein, D.; Alvarez, V.A.; Amarasingham, A.; Azab, H.; Chen, Z.S.; Gerkin, R.C.; Hasenstaub, A.; Iyer, R.; Jolivet, R.B.; Marzen, S.; et al. On the role of theory and modeling in neuroscience. J. Neurosci. 2023, 43, 1074–1088. [Google Scholar] [CrossRef]
- Santamaría-Vázquez, E.; Martínez-Cagigal, V.; Marcos-Martínez, D.; Rodríguez-González, V.; Pérez-Velasco, S.; Moreno-Calderón, S.; Hornero, R. MEDUSA©: A novel Python-based software ecosystem to accelerate brain-computer interface and cognitive neuroscience research. Comput. Methods Programs Biomed. 2023, 230, 107357. [Google Scholar] [CrossRef]
- Laerum, O.D.; Steinsvåg, S.; Bjerkvig, R. Cell and tissue culture of the central nervous system: Recent developments and current applications. Acta Neurol. Scand. 1985, 72, 529–549. [Google Scholar] [CrossRef]
- Wu, V.W.; Schwartz, J.P. Cell culture models for reactive gliosis: New perspectives. J. Neurosci. Res. 1998, 51, 675–681. [Google Scholar] [CrossRef]
- Gibbons, H.M.; Dragunow, M. Adult human brain cell culture for neuroscience research. Int. J. Biochem. Cell Biol. 2010, 42, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. Reality check for organoids in neuroscience. Nat. Methods 2020, 17, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, M.; Zhou, J. Brain organoids: A promising living biobank resource for neuroscience research. Biopreserv. Biobank. 2020, 18, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Sun, W. Neural organoids, a versatile model for neuroscience. Mol. Cells 2022, 45, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Bayne, K.; Turner, P.V. Animal welfare standards and international collaborations. ILAR J. 2019, 60, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Medina, L.V. building a culture of animal welfare: Past, present and future. Annu. Rev. Biomed. Sci. 2008, 10, 104–111. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montanari, M.; Bonsi, P.; Martella, G.; Wirz, A. Animal Models in Neuroscience: What Is the “Culture of Care”? Encyclopedia 2024, 4, 215-233. https://0-doi-org.brum.beds.ac.uk/10.3390/encyclopedia4010018
Montanari M, Bonsi P, Martella G, Wirz A. Animal Models in Neuroscience: What Is the “Culture of Care”? Encyclopedia. 2024; 4(1):215-233. https://0-doi-org.brum.beds.ac.uk/10.3390/encyclopedia4010018
Chicago/Turabian StyleMontanari, Martina, Paola Bonsi, Giuseppina Martella, and Annarita Wirz. 2024. "Animal Models in Neuroscience: What Is the “Culture of Care”?" Encyclopedia 4, no. 1: 215-233. https://0-doi-org.brum.beds.ac.uk/10.3390/encyclopedia4010018