Sublimation Study of Six 5-Substituted-1,10-Phenanthrolines by Knudsen Effusion Mass Loss and Solution Calorimetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Instruments
2.2.1. DSC Measurements
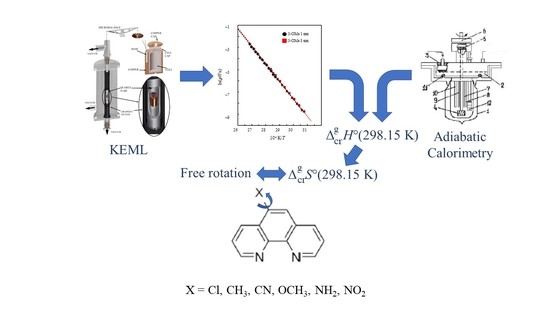
2.2.2. KEML Measurements
2.2.3. Solution Calorimetry Measurements
3. Results and Discussion
3.1. Melting Parameters Determination by DSC Experiments
3.2. Vapor Pressure Determination by KEML Experiments
3.3. Sublimation Enthalpies of 1,10-Phenanthrolines Obtained by the Solution Calorimetry Approach
3.4. Standard Molar Sublimation Enthalpies
ΔcrgHm0(⟨T⟩) + [ΔcrgCp,m0/J·K−1·mol−1]·(⟨T⟩/K − 298.15)/1000,
ΔcrgCp,m0/J·K−1·mol−1 · ln[(298.15 K/⟨T⟩)] − R·ln(p0/p⟨T⟩)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sammes, P.G.; Yahioglu, G. 1,10-Phenanthroline: A versatile ligand. Chem. Soc. Rev. 1994, 23, 299–362. [Google Scholar] [CrossRef]
- Wu, F.; Xie, J.; Zhu, Z. 1,10-Phenanthroline: A versatile ligand to promote copper-catalyzed cascade reactions. Appl. Organomet. Chem. 2020, 34, e5926. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.; Kumar, A.; Murthy, K.S.R.; Singh, A.K. Ruthenium(II) photosensitizers bearing imidazo [4,5-f][1,10] phenanthroline scaffolds. Coord. Chem. Rev. 2021, 452, 214272. [Google Scholar] [CrossRef]
- Rentschler, M.; Schmid, M.-A.; Frey, W.; Tschierlei, S.; Karnahl, M. Multidentate Phenanthroline Ligands Containing Additional Donor Moieties and Their Resulting Cu(I) and Ru(II) Photosensitizers: A Comparative Study. Inorg. Chem. 2020, 59, 14762–14771. [Google Scholar] [CrossRef]
- Masuri, S.; Vaňhara, P.; Cabiddu, M.G.; Moráň, L.; Havel, J.; Cadoni, E.; Pivetta, T. Copper(II) Phenanthroline-Based Complexes as Potential AntiCancer Drugs: A Walkthrough on the Mechanisms of Action. Molecules 2022, 27, 49. [Google Scholar] [CrossRef]
- Sánchez-González, Á.; Bandeira, N.A.G.; Ortiz de Luzuriaga, I.; Martins, F.F.; Elleuchi, S.; Jarraya, K.; Lanuza, J.; Lopez, X.; Calhorda, M.J.; Gil, A. New Insights on the Interaction of Phenanthroline Based Ligands and Metal Complexes and Polyoxometalates with Duplex DNA and G-Quadruplexes. Molecules 2021, 26, 4737. [Google Scholar] [CrossRef]
- Bonicelli, M.G.; Catalani, A.; Mariano, G.; Vecchio, S. Heat capacities, and molar enthalpies and entropies of fusion for anhydrous 1,10-phenanthroline and 2,9-dimethyl-1,10-phenanthroline. Thermochim. Acta 2007, 466, 67–71. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Chartrand, P. Critical assessment of thermodynamic properties of important polycyclic aromatic hydrocarbon compounds (PAHs) in coal tar pitch at typical temperature ranges of the carbonization process. Calphad 2021, 74, 102278. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Campos, R.M.; Lima, L.M.S.S.; Ribeiro da Silva, M.A.V.; Santos, L.M.B.F. On the Aromatic Stabilization of Fused Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. A 2021, 125, 3696–3709. [Google Scholar] [CrossRef]
- Goldfarb, J.L. Review of Sublimation Thermodynamics of Polycyclic Aromatic Compounds and Heterocycles. J. Heterocycl. Chem. 2013, 50, 1243–1263. [Google Scholar] [CrossRef]
- Belova, N.V.; Giricheva, N.I.; Zhabanov, Y.A.; Andreev, V.P.; Girichev, G.V. Sublimation Enthalpies of Substituted Pyridine N-Oxides. Russ. J. Gen. Chem. 2021, 91, 1932–1937. [Google Scholar] [CrossRef]
- Brunetti, B.; Lapi, A.; Vecchio Ciprioti, S. Thermodynamic study on six tricyclic nitrogen heterocyclic compounds by thermal analysis and effusion techniques. Thermochim. Acta 2016, 636, 71–84. [Google Scholar] [CrossRef]
- Chirico, R.D.; Kazakov, A.F.; Steele, W.V. Thermodynamic properties of three-ring aza-aromatics. 1. Experimental results for phenazine and acridine, and mutual validation of experiments and computational methods. J. Chem. Thermodyn. 2010, 42, 571–580. [Google Scholar] [CrossRef]
- Chirico, R.D.; Kazakov, A.F.; Steele, W.V. Thermodynamic properties of three-ring aza-aromatics. 2. Experimental results for 1,10-phenanthroline, phenanthridine, and 7,8-benzoquinoline, and mutual validation of experiments and computational methods. J. Chem. Thermodyn. 2010, 42, 581–590. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Varfolomeev, M.A.; Nagrimanov, R.N.; Novikov, V.B.; Buzyurov, A.V.; Fedorova, Y.V.; Mukhametzyanov, T.A. New method for determination of vaporization and sublimation enthalpy of aromatic compounds at 298.15K using solution calorimetry technique and group-additivity scheme. Thermochim. Acta 2015, 622, 88–96. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Nagrimanov, R.N.; Mukhametzyanov, T.A. Additive scheme for calculation of solvation enthalpies of heterocyclic aromatic compounds. Sublimation/vaporization enthalpy at 298.15 K. Thermochim. Acta 2016, 633, 37–47. [Google Scholar] [CrossRef]
- Vecchio, S.; Brunetti, B. Thermochemical study of 2,4-, 2,6- and 3,4-dihydroxybenzoic acids in the liquid phase using a TG apparatus. Thermochim. Acta 2011, 515, 84–90. [Google Scholar] [CrossRef]
- Vecchio, S.; Brunetti, B. Vapor Pressures and Standard Molar Sublimation Enthalpies of three 6-methylthio-2,4-di(alkylamino)-1,3,5-Triazine Derivatives: Simetryn, Ametryn and Terbutryn. J. Chem. Eng. Data 2007, 52, 1585–1594. [Google Scholar] [CrossRef]
- Shen, Y.; Sullivan, B.P. A Versatile Preparative Route to 5-Substituted-1,10-Phenanthroline Ligands via 1,10-Phenanthroline 5,6-Epoxide. Inorg. Chem. 1995, 34, 6235–6236. [Google Scholar] [CrossRef]
- Ji, S.; Guo, H.; Yuan, X.; Li, X.; Ding, H.; Gao, P.; Zhao, C.; Wu, W.; Wu, W.; Zhao, J. A Highly Selective Off-On red emitting Phosphorescent Thiol Probe with Large Stokes Shift and Long Luminescent Lifetime. Org. Lett. 2010, 12, 2876–2879. [Google Scholar] [CrossRef]
- Sabbah, R.; Xu-wu, A.; Chickos, J.S.; Planas Leitão, M.L.; Roux, M.V.; Torres, L.A. Reference materials for calorimetry and differential thermal analysis. Thermochim. Acta 1999, 331, 93–204. [Google Scholar] [CrossRef]
- Della Gatta, G.; Richardson, M.J.; Sarge, S.M.; Stolen, S. Standards, calibration, and guidelines in microcalorimetry. Part 2. Calibration standards for differential scanning calorimetry (IUPAC Technical Report). Pure Appl. Chem. 2006, 78, 1455–1476. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, B.; Ciccioli, A.; Gigli, G.; Lapi, A.; Misceo, N.; Tanzi, L.; Vecchio Ciprioti, S. Vaporization of the prototypical ionic liquid BMImNTf2 under equilibrium conditions: A multitechnique study. Phys. Chem. Chem. Phys. 2014, 16, 15653–15661. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, M. Experimentelle Bestimmung des Druckes gesattiger Quecksilberdampfebei 0° und hiheren Temperaturen. Ann. Physik 1909, 29, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Monte, M.J.S.; Santos, L.M.N.B.F.; Fulem, M.; Fonseca, J.M.S.; Sousa, C.A.D. New static apparatus and vapor pressure of reference materials: Naphthalene, benzoic acid, benzophenone, and ferrocene. J. Chem. Eng. Data 2006, 51, 757–766. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Monte, M.J.S.; Santos, L.M.N.B.F. The design, construction, and testing of a new Knudsen effusion apparatus. J. Chem. Thermodyn. 2006, 38, 778–787. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Novikov, V.B.; Nagrimanov, R.N.; Solomonov, B.N. Modified solution calorimetry approach for determination of vaporization and sublimation enthalpies of branched-chain aliphatic and alkyl aromatic compounds at T = 298.15 K. J. Chem. Thermodyn. 2015, 91, 204–210. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Nagrimanov, R.N.; Varfolomeev, M.A.; Buzyurov, A.V.; Mukhametzyanov., T.A. Enthalpies of fusion and enthalpies of solvation of aromatic hydrocarbons derivatives: Estimation of sublimation enthalpies at 298.15 K. Thermochim. Acta 2016, 627, 77–82. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hosseini, S.; Hesse, D.G.; Liebman, J.F. Heat capacity corrections to a standard state: A comparison of new and some literature methods for organic liquids and solids. Struct. Chem. 1993, 4, 271–277. [Google Scholar] [CrossRef]
- Acree, W., Jr.; Chickos, J.S. Phase transition enthalpy measurements of organic and organometallic compounds. Sublimation, vaporization and fusion enthalpies from 1880 to 2010. J. Phys. Chem. Ref. Data 2010, 39, 043101. [Google Scholar] [CrossRef]
- Acree, W., Jr.; Chickos, J.S. Phase transition enthalpy measurements of organic and organometallic compounds. Sublimation, vaporization and fusion enthalpies from 1880 to 2015. Part 1. C1–C10. J. Phys. Chem. Ref. Data 2016, 45, 033101. [Google Scholar] [CrossRef]
- Acree, W., Jr.; Chickos, J.S. Enthalpies of sublimation of organic and organometallic compounds. 1910–2001. J. Phys. Chem. Ref. Data 2002, 32, 537–698. [Google Scholar] [CrossRef]
- Fukuyo, M.; Hirotsu, K.; Higuchi, T. The Structure of Aniline at 252 K. Acta Crystallogr. 1982, B38, 640–643. [Google Scholar] [CrossRef]
- Page, M.I.; Jencks, W.P. Entropic Contributions to Rate Accelerations in Enzymic and Intramolecular Reactions and the Chelate Effect. Proc. Natl. Acad. Sci. USA 1971, 68, 1678–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Compound | Source | Purification Method | Final Mass Fraction Purity | Analysis Method | Water Content/ppm |
|---|---|---|---|---|---|
| 5-Cl-1,10-phenanthroline | Sigma-Aldrich | - | >0.98 | - | 50 |
| 5-CH3-1,10-phenanthroline | Sigma-Aldrich | - | >0.99 | - | 30 |
| 5-CH3O-1,10-phenanthroline | Synthesis | Chromatography | >0.999 | GC | - |
| 5-CN-1,10-phenanthroline | Synthesis | Recrystallization | >0.999 | GC | - |
| 5-NO2-1,10-phenanthroline | Synthesis | Recrystallization | >0.999 | GC | - |
| 5-NH2-1,10-phenanthroline | Synthesis | Recrystallization | >0.999 | GC | - |
| benzene | Ekos-1 | Distillation | >0.999 | GC | 20 |
| Compound | Tfusa | ΔcrlHm0 b |
|---|---|---|
| K | kJ·mol−1 | |
| 5-Cl-1,10-phenanthroline | 396.5 ± 0.2 | 18.9 ± 0.6 |
| 5-CH3-1,10-phenanthroline | 384.3 ± 0.2 | 8.9 ± 0.4 |
| 5-CH3O-1,10-phenanthroline | ||
| 5-CN-1,10-phenanthroline | ||
| 5-NO2-1,10-phenanthroline | 473.0 ± 0.2 | 25.2 ± 0.8 |
| 5-NH2-1,10-phenanthroline | 525.1 ± 0.2 | 24.1 ± 0.8 |
| T/K | Δt/s | Δm/mg a | p/Pa a | 100 Δp/p b | T/K | Δt/s | Δm/mg a | p/Pa a | 100 Δp/p b |
|---|---|---|---|---|---|---|---|---|---|
| 5-chloro-1,10-phenanthroline | |||||||||
| ∮/mm = 1 | ∮/mm = 3 | ||||||||
| 367.1 | 4074 | 1.20 | 0.206 | −3.7 | 323.1 | 15,821 | 0.19 | 0.00152 | −2.6 |
| 361.3 | 4430 | 0.77 | 0.121 | 2.7 | 312.9 | 40,125 | 0.14 | 0.000437 | 7.8 |
| 355.4 | 4593 | 0.42 | 0.0626 | −2.0 | 332.7 | 6951 | 0.29 | 0.00535 | 3.7 |
| 349.6 | 6239 | 0.30 | 0.0328 | −4.0 | 327.7 | 14,246 | 0.31 | 0.00278 | −0.3 |
| 343.8 | 9256 | 0.23 | 0.0167 | −6.0 | 322.6 | 24,318 | 0.29 | 0.00153 | 4.4 |
| 338.0 | 21,233 | 0.29 | 0.00907 | 0.8 | 317.8 | 27,704 | 0.17 | 0.000784 | 0.9 |
| 332.2 | 31,710 | 0.23 | 0.00479 | 6.2 | 312.9 | 32,694 | 0.11 | 0.000424 | 4.8 |
| 365.2 | 5447 | 1.39 | 0.178 | 1.0 | 308.0 | 96,302 | 0.14 | 0.000186 | −10.0 |
| 359.3 | 4836 | 0.67 | 0.0963 | 0.4 | 339.6 | 7394 | 0.65 | 0.0115 | −1.0 |
| 353.6 | 4720 | 0.37 | 0.0537 | 2.7 | 337.6 | 6903 | 0.48 | 0.00903 | −2.5 |
| 347.7 | 9264 | 0.37 | 0.0274 | −0.2 | 334.7 | 9694 | 0.47 | 0.00624 | −5.2 |
| 341.9 | 12,381 | 0.26 | 0.0142 | −0.3 | 329.8 | 15,100 | 0.46 | 0.00389 | 6.4 |
| 336.1 | 24,654 | 0.26 | 0.00705 | −1.8 | 325.0 | 19,951 | 0.30 | 0.00192 | −3.4 |
| 363.3 | 5313 | 1.15 | 0.151 | 4.1 | 320.1 | 37,451 | 0.31 | 0.00104 | −2.7 |
| 357.4 | 4453 | 0.51 | 0.0796 | 0.9 | 315.3 | 52,795 | 0.23 | 0.000552 | −0.9 |
| 351.6 | 6174 | 0.38 | 0.0419 | −0.9 | 309.8 | 72,493 | 0.15 | 0.000260 | −1.1 |
| 345.8 | 12,210 | 0.41 | 0.0227 | 2.5 | |||||
| 339.9 | 27,239 | 0.44 | 0.0108 | −5.0 | |||||
| 334.1 | 58,330 | 0.50 | 0.00577 | 1.5 | |||||
| 5-methyl-1,10-phenanthroline | |||||||||
| ∮/mm = 1 | ∮/mm = 3 | ||||||||
| 372.3 | 5983 | 2.72 | 0.373 | 2.9 | 337.0 | 9042 | 0.59 | 0.0107 | 2.1 |
| 368.4 | 4837 | 1.48 | 0.250 | −0.3 | 334.0 | 8058 | 0.37 | 0.00749 | 0.7 |
| 364.5 | 4987 | 1.03 | 0.168 | −2.2 | 331.1 | 10,234 | 0.33 | 0.00516 | −1.8 |
| 360.5 | 5436 | 0.80 | 0.119 | 2.2 | 328.1 | 13,521 | 0.31 | 0.00365 | −1.4 |
| 356.6 | 5022 | 0.49 | 0.0785 | 0.0 | 325.2 | 22,546 | 0.35 | 0.00252 | −2.4 |
| 352.7 | 6864 | 0.47 | 0.0542 | 2.7 | 322.2 | 21,835 | 0.25 | 0.00179 | 0.0 |
| 348.8 | 9740 | 0.44 | 0.0361 | 3.7 | 319.3 | 48,156 | 0.39 | 0.00128 | 3.3 |
| 344.7 | 17,221 | 0.48 | 0.0220 | −2.6 | 316.3 | 66,072 | 0.35 | 0.000837 | −1.4 |
| 340.8 | 18,658 | 0.33 | 0.0141 | −4.1 | 311.6 | 244,549 | 0.69 | 0.000440 | −3.9 |
| 370.3 | 5857 | 2.15 | 0.301 | −0.1 | 338.9 | 7466 | 0.59 | 0.0128 | −1.3 |
| 366.4 | 4954 | 1.25 | 0.206 | −0.8 | 335.0 | 5878 | 0.29 | 0.00817 | −1.5 |
| 362.4 | 4814 | 0.86 | 0.144 | 2.8 | 332.0 | 7950 | 0.29 | 0.00585 | −0.5 |
| 358.5 | 6889 | 0.82 | 0.0955 | 0.4 | 329.1 | 12,120 | 0.31 | 0.00417 | 0.6 |
| 354.6 | 6808 | 0.51 | 0.0604 | −6.0 | 326.1 | 19,705 | 0.34 | 0.00275 | −4.8 |
| 350.7 | 9825 | 0.50 | 0.0403 | −5.8 | 323.1 | 20,423 | 0.28 | 0.00219 | 8.4 |
| 346.8 | 12,692 | 0.45 | 0.0283 | 0.0 | 320.2 | 42,259 | 0.37 | 0.00139 | 0.4 |
| 342.9 | 15,825 | 0.37 | 0.0186 | 0.8 | 317.2 | 57,261 | 0.36 | 0.00101 | 5.6 |
| 338.9 | 22,030 | 0.35 | 0.0125 | 5.0 | 314.3 | 87,374 | 0.36 | 0.000646 | −0.8 |
| 335.0 | 39,950 | 0.37 | 0.00726 | −4.5 | 311.4 | 139,369 | 0.38 | 0.000431 | −2.3 |
| 331.0 | 54,412 | 0.35 | 0.00504 | 5.0 | |||||
| 5-methoxy-1,10-phenanthroline | |||||||||
| ∮/mm = 1 | ∮/mm = 3 | ||||||||
| 372.3 | 6709 | 1.52 | 0.183 | 0.6 | 352.6 | 6056 | 0.90 | 0.0194 | 4.5 |
| 368.4 | 4712 | 0.70 | 0.119 | 2.9 | 348.4 | 9816 | 0.86 | 0.0113 | 5.5 |
| 364.5 | 4606 | 0.43 | 0.0757 | 3.7 | 344.4 | 4851 | 0.25 | 0.00658 | 3.7 |
| 360.4 | 6832 | 0.39 | 0.0456 | 2.1 | 340.6 | 6547 | 0.20 | 0.00390 | 2.8 |
| 356.3 | 9829 | 0.35 | 0.0285 | 5.4 | 336.7 | 10,399 | 0.18 | 0.00225 | 2.3 |
| 352.3 | 15,280 | 0.33 | 0.0168 | 3.4 | 332.8 | 27,497 | 0.29 | 0.00134 | 5.5 |
| 348.5 | 40,339 | 0.51 | 0.00984 | 0.0 | 328.9 | 48,497 | 0.29 | 0.000758 | 5.1 |
| 370.7 | 5230 | 0.93 | 0.144 | −4.5 | 325.0 | 111,603 | 0.39 | 0.000434 | 7.2 |
| 366.7 | 4490 | 0.52 | 0.0930 | −2.5 | 350.4 | 5903 | 0.61 | 0.0134 | −3.1 |
| 362.3 | 6220 | 0.42 | 0.0544 | −2.6 | 346.5 | 4992 | 0.31 | 0.00801 | −3.7 |
| 358.3 | 9393 | 0.40 | 0.0335 | −2.5 | 342.6 | 6606 | 0.24 | 0.00471 | −4.7 |
| 354.3 | 15,204 | 0.40 | 0.0208 | −0.6 | 338.7 | 15,350 | 0.32 | 0.00268 | −7.9 |
| 350.4 | 24,988 | 0.38 | 0.0120 | −6.1 | 334.5 | 31,848 | 0.37 | 0.00148 | −9.0 |
| 330.6 | 50,156 | 0.34 | 0.000865 | −6.2 | |||||
| 326.7 | 48,936 | 0.19 | 0.000496 | −4.0 | |||||
| 5-cyano-1,10-phenanthroline | |||||||||
| ∮/mm = 1 | ∮/mm = 3 | ||||||||
| 413.7 | 4686 | 4.33 | 0.799 | 1.5 | 376.4 | 6387 | 1.01 | 0.0217 | 6.4 |
| 409.8 | 4660 | 2.80 | 0.518 | −5.7 | 372.4 | 4140 | 0.43 | 0.0141 | 7.6 |
| 405.9 | 4651 | 2.10 | 0.387 | 3.1 | 368.5 | 5050 | 0.32 | 0.00865 | 3.3 |
| 401.9 | 4673 | 1.43 | 0.261 | 2.1 | 364.7 | 8911 | 0.37 | 0.00552 | 2.5 |
| 398.1 | 4737 | 0.99 | 0.177 | 1.4 | 360.8 | 17,601 | 0.46 | 0.00349 | 2.8 |
| 394.2 | 4736 | 0.67 | 0.119 | 0.8 | 357.0 | 21,827 | 0.37 | 0.00226 | 6.1 |
| 390.3 | 4388 | 0.42 | 0.0801 | 1.3 | 353.0 | 39,028 | 0.37 | 0.00127 | −2.6 |
| 386.3 | 6370 | 0.40 | 0.0527 | 1.0 | 349.2 | 69,135 | 0.43 | 0.000810 | 1.6 |
| 382.5 | 9638 | 0.38 | 0.0327 | −5.8 | 345.3 | 11,8495 | 0.42 | 0.000463 | −4.6 |
| 378.7 | 19,865 | 0.57 | 0.0236 | 3.6 | 378.3 | 6794 | 1.16 | 0.0234 | −7.7 |
| 408.3 | 5201 | 2.84 | 0.468 | −1.0 | 374.4 | 5730 | 0.68 | 0.0161 | −1.4 |
| 404.3 | 4650 | 1.71 | 0.314 | −3.1 | 370.6 | 4833 | 0.36 | 0.0101 | −4.5 |
| 402.0 | 5241 | 1.49 | 0.242 | −6.6 | 366.6 | 9197 | 0.46 | 0.00680 | 1.1 |
| 398.0 | 4350 | 0.84 | 0.164 | −5.8 | 362.7 | 17,937 | 0.56 | 0.00421 | −1.0 |
| 400.5 | 5104 | 1.31 | 0.219 | −2.2 | 358.9 | 21,220 | 0.41 | 0.00257 | −4.1 |
| 396.5 | 4545 | 0.79 | 0.147 | −1.9 | 355.0 | 38,864 | 0.48 | 0.00164 | −1.6 |
| 392.7 | 4333 | 0.51 | 0.0991 | −2.5 | 351.1 | 69,000 | 0.52 | 0.00100 | −2.5 |
| 388.8 | 4513 | 0.36 | 0.0665 | −2.3 | 347.2 | 94,468 | 0.43 | 0.000598 | −4.0 |
| 384.8 | 6556 | 0.34 | 0.0430 | −3.2 | 377.1 | 4614 | 0.74 | 0.0218 | −0.6 |
| 381.0 | 9294 | 0.32 | 0.0285 | −3.0 | 369.4 | 4526 | 0.30 | 0.00909 | −2.0 |
| 377.1 | 18,437 | 0.45 | 0.0203 | 5.8 | 373.3 | 6353 | 0.65 | 0.0140 | −2.4 |
| 408.5 | 5599 | 3.18 | 0.488 | 1.1 | 365.6 | 8871 | 0.38 | 0.00584 | −1.8 |
| 404.6 | 5152 | 2.07 | 0.344 | 3.1 | 361.7 | 18,077 | 0.49 | 0.00363 | −2.9 |
| 400.8 | 5450 | 1.62 | 0.253 | 8.9 | 357.8 | 21,203 | 0.39 | 0.00247 | 4.8 |
| 396.9 | 4550 | 0.89 | 0.165 | 5.5 | 353.9 | 39,299 | 0.40 | 0.00135 | −7.1 |
| 393.1 | 5393 | 0.70 | 0.110 | 4.1 | 350.0 | 68,371 | 0.50 | 0.000967 | 8.9 |
| 389.2 | 5304 | 0.46 | 0.0725 | 2.7 | 346.0 | 72,267 | 0.30 | 0.000551 | 3.4 |
| 385.4 | 8506 | 0.46 | 0.0454 | −3.9 | |||||
| 381.4 | 6767 | 0.25 | 0.0306 | −1.1 | |||||
| 5-nitro-1,10-phenanthroline | |||||||||
| ∮/mm = 1 | ∮/mm = 3 | ||||||||
| 411.9 | 4784 | 4.41 | 0.638 | 1.5 | 392.2 | 3973 | 2.93 | 0.100 | −2.5 |
| 408.9 | 3676 | 2.55 | 0.480 | −0.1 | 387.1 | 4240 | 1.92 | 0.0612 | 0.9 |
| 405.9 | 4039 | 2.15 | 0.366 | 1.4 | 382.6 | 2480 | 0.71 | 0.0382 | 2.0 |
| 403.0 | 4165 | 1.68 | 0.276 | 0.4 | 377.7 | 4454 | 0.75 | 0.0225 | 2.7 |
| 399.9 | 4172 | 1.25 | 0.204 | −0.4 | 372.3 | 2679 | 0.25 | 0.0123 | 3.0 |
| 396.9 | 4283 | 0.95 | 0.151 | −0.7 | 367.3 | 10,640 | 0.56 | 0.00698 | 3.5 |
| 394.3 | 4363 | 0.74 | 0.115 | −1.5 | 362.4 | 20,845 | 0.61 | 0.00382 | 1.3 |
| 391.0 | 4053 | 0.50 | 0.0834 | −1.1 | 357.7 | 44,081 | 0.72 | 0.00212 | −0.4 |
| 389.0 | 4737 | 0.48 | 0.0685 | −0.7 | 352.6 | 141,123 | 1.24 | 0.00113 | 0.7 |
| 413.2 | 4482 | 4.60 | 0.712 | 0.0 | 390.1 | 4700 | 2.85 | 0.0821 | −0.8 |
| 410.2 | 4535 | 3.55 | 0.540 | −0.3 | 385.2 | 4181 | 1.50 | 0.0485 | −1.9 |
| 407.3 | 4522 | 2.73 | 0.416 | 0.9 | 380.1 | 4440 | 0.97 | 0.0291 | 1.9 |
| 404.5 | 4632 | 2.15 | 0.319 | 1.2 | 375.2 | 3939 | 0.50 | 0.0170 | 2.4 |
| 401.5 | 4598 | 1.59 | 0.237 | −0.3 | 370.3 | 6481 | 0.47 | 0.00962 | 1.1 |
| 398.5 | 4622 | 1.20 | 0.177 | −0.4 | 365.4 | 12,680 | 0.51 | 0.00524 | −3.2 |
| 395.6 | 4524 | 0.89 | 0.134 | 0.7 | 360.4 | 21,482 | 0.45 | 0.00274 | −7.7 |
| 392.6 | 4533 | 0.68 | 0.101 | 1.4 | 355.4 | 71,928 | 0.93 | 0.00168 | 4.9 |
| 390.6 | 4526 | 0.54 | 0.0807 | −0.5 | 388.7 | 3640 | 1.89 | 0.0703 | −1.8 |
| 418.4 | 4373 | 7.15 | 1.14 | 0.4 | 383.8 | 4280 | 1.34 | 0.0421 | −1.0 |
| 416.5 | 4213 | 5.82 | 0.961 | 0.4 | 378.9 | 4161 | 0.76 | 0.0245 | −2.1 |
| 415.0 | 4283 | 5.21 | 0.846 | 1.2 | 374.0 | 4320 | 0.47 | 0.0145 | −0.7 |
| 424.1 | 4333 | 11.01 | 1.79 | −4.6 | 369.1 | 6880 | 0.45 | 0.00863 | 3.9 |
| 420.7 | 3227 | 6.45 | 1.40 | 1.0 | 364.2 | 12,920 | 0.47 | 0.00472 | 1.1 |
| 417.8 | 3762 | 5.78 | 1.07 | −0.1 | 359.3 | 35,846 | 0.66 | 0.00238 | −8.2 |
| 5-amino-1,10-phenanthroline | |||||||||
| ∮/mm = 1 | ∮/mm = 3 | ||||||||
| 441.9 | 1731 | 0.92 | 0.407 | −0.5 | 402.4 | 6685 | 0.44 | 0.00979 | 2.7 |
| 438.7 | 3251 | 1.32 | 0.312 | 0.9 | 399.3 | 6935 | 0.33 | 0.00706 | 4.6 |
| 436.3 | 4643 | 1.54 | 0.254 | 2.3 | 396.6 | 8429 | 0.30 | 0.00519 | 3.4 |
| 433.6 | 1920 | 0.50 | 0.200 | 3.2 | 393.7 | 10,468 | 0.27 | 0.00375 | 3.6 |
| 427.6 | 4411 | 0.66 | 0.113 | 2.1 | 390.5 | 18,821 | 0.32 | 0.00245 | −2.9 |
| 421.0 | 5136 | 0.40 | 0.0584 | 0.2 | 387.1 | 28,236 | 0.33 | 0.00168 | −1.9 |
| 418.3 | 5221 | 0.31 | 0.0449 | 0.7 | 384.4 | 45,736 | 0.42 | 0.00132 | 5.3 |
| 415.4 | 7575 | 0.32 | 0.0319 | −5.4 | 381.4 | 158,054 | 1.01 | 0.000919 | 5.6 |
| 412.1 | 4566 | 0.14 | 0.0229 | −4.5 | 403.6 | 2880 | 0.21 | 0.0106 | −2.0 |
| 409.0 | 6392 | 0.17 | 0.0193 | 9.5 | 400.9 | 3242 | 0.17 | 0.00775 | −3.5 |
| 406.1 | 23,930 | 0.40 | 0.0123 | −5.5 | 397.6 | 7321 | 0.28 | 0.00567 | 1.3 |
| 403.3 | 24,877 | 0.31 | 0.00906 | −5.3 | 394.6 | 13,323 | 0.36 | 0.00393 | −1.8 |
| 440.9 | 1680 | 0.81 | 0.373 | −0.7 | 391.8 | 18,483 | 0.35 | 0.00277 | −5.1 |
| 438.1 | 1500 | 0.56 | 0.286 | −1.4 | 388.7 | 36,843 | 0.49 | 0.00195 | −5.5 |
| 434.7 | 2721 | 0.75 | 0.210 | −2.3 | 385.8 | 54,628 | 0.55 | 0.00146 | −0.5 |
| 431.9 | 1540 | 0.33 | 0.161 | −2.2 | 383.9 | 69,853 | 0.54 | 0.00112 | −4.3 |
| 429.0 | 1400 | 0.23 | 0.123 | −2.1 | |||||
| 425.9 | 1680 | 0.20 | 0.0920 | −2.4 | |||||
| 423.2 | 1521 | 0.14 | 0.0706 | −2.5 | |||||
| 416.9 | 3481 | 0.19 | 0.0416 | 6.8 | |||||
| 413.9 | 6120 | 0.25 | 0.0304 | 5.2 | |||||
| 410.4 | 8611 | 0.24 | 0.0203 | 1.2 | |||||
| 407.7 | 15,540 | 0.32 | 0.0154 | 0.8 | |||||
| 405.1 | 24,605 | 0.39 | 0.0116 | 0.1 | |||||
| Compound | ΔsolnHmAi/S a | ΔsolvHmAi/S b | ΔcrgHm0 c |
|---|---|---|---|
| kJ·mol−1 | kJ·mol−1 | kJ·mol−1 | |
| 5-Cl-1,10-phenanthroline (cr) | 19.39 ± 0.11 | 91.5 ± 1.0 | 110.9 ± 1.0 |
| 5-CH3-1,10-phenanthroline (cr) | 18.34 ± 0.20 | 88.9 ± 1.0 | 107.2 ± 1.0 |
| Compound | ΔT/K | ln(p/Pa) = A − B/T | ||
|---|---|---|---|---|
| Aa | B/K a | OD b, ∮/mm | ||
| 5-Cl-1,10-phenanthroline | 332.2–367.1 | 35.150 ± 0.245 | 13472 ± 85 | 1 |
| 308.0–339.6 | 34.986 ± 0.401 | 13392 ± 129 | 3 | |
| 5-CH3-1,10-phenanthroline | 331.0–372.3 | 33.691 ± 0.227 | 12921 ± 80 | 1 |
| 311.4–338.9 | 33.932 ± 0.296 | 12971 ± 96 | 3 | |
| 5-CH3O-1,10-phenanthroline | 348.5–372.3 | 40.905 ± 0.480 | 15864 ± 173 | 1 |
| 325.0–352.6 | 41.114 ± 0.587 | 15904 ± 199 | 3 | |
| 5-CN-1,10-phenanthroline | 377.1–413.7 | 38.031 ± 0.290 | 15833 ± 115 | 1 |
| 345.3–378.3 | 37.624 ± 0.313 | 15624 ± 113 | 3 | |
| 5-NO2-1,10-phenanthroline | 389.0–424.1 | 37.259 ± 0.112 | 15536 ± 45 | 1 |
| 352.6–392.2 | 38.004 ± 0.214 | 15796 ± 80 | 3 | |
| 5- NH2-1,10-phenanthroline | 403.3–441.9 | 38.349 ± 0.274 | 17341 ± 116 | 1 |
| 381.4–403.6 | 38.736 ± 0.574 | 17462 ± 225 | 3 | |
| Compound | Method, OD | ⟨T⟩/K | ΔcrgHm0(⟨T⟩) | Cp,m0 (cr) b | −ΔcrgCp,m0 | ΔcrgH m0 (298.15 K) c |
|---|---|---|---|---|---|---|
| kJ·mol−1 | J·K−1·mol−1 | J·K−1·mol−1 | kJ·mol−1 | |||
| 5-Cl-1,10-phenanthroline | KEML, 1 mm | 349.6 | 112.0 ± 0.7 | 221.5 | 34.0 | 113.8 ± 0.9 |
| KEML, 3 mm | 323.1 | 111.4 ± 1.1 | 221.5 | 34.0 | 112.2 ± 1.1 | |
| average | 113.0 ± 0.7 | |||||
| SC a | 110.9 ± 1.0 | |||||
| recommended | 112.0 ± 0.8 | |||||
| 5-CH3-1,10-phenanthroline | KEML, 1 mm | 353.2 | 107.4 ± 0.7 | 229.4 | 35.2 | 109.4 ± 0.9 |
| KEML, 3 mm | 324.8 | 107.8 ± 0.8 | 229.4 | 35.2 | 108.8 ± 0.9 | |
| average | 109.1 ± 0.6 | |||||
| SC a | 107.2 ± 1.0 | |||||
| recommended | 108.2 ± 0.8 | |||||
| 5-CH3O-1,10-phenanthroline | KEML, 1 mm | 360.4 | 131.9 ± 1.4 | 279.2 | 42.6 | 134.6 ± 1.7 |
| KEML, 3 mm | 338.6 | 132.2 ± 1.7 | 279.2 | 42.6 | 134.0 ± 1.8 | |
| average | 134.3 ± 1.2 | |||||
| recommended | 134.3 ± 1.2 | |||||
| 5-CN-1,10-phenanthroline | KEML, 1 mm | 395.0 | 131.6 ± 1.0 | 235.1 | 36.0 | 135.1 ± 1.5 |
| KEML, 3 mm | 361.7 | 129.9 ± 0.9 | 235.1 | 36.0 | 132.2 ± 1.2 | |
| average | 133.7 ± 1.0 | |||||
| recommended | 133.7 ± 1.0 | |||||
| 5-NO2-1,10-phenanthroline | KEML, 1 mm | 405.3 | 129.2 ± 0.4 | 248.9 | 38.1 | 133.3 ± 1.4 |
| KEML, 3 mm | 373.0 | 131.3 ± 0.7 | 248.9 | 38.1 | 134.2 ± 1.2 | |
| average | 133.7 ± 0.9 | |||||
| recommended | 133.7 ± 0.9 | |||||
| 5- NH2-1,10-phenanthroline | KEML, 1 mm | 422.5 | 144.2 ± 1.0 | 214.4 | 32.9 | 148.3 ± 1.7 |
| KEML, 3 mm | 392.6 | 145.2 ± 1.9 | 214.4 | 32.9 | 148.3 ± 2.1 | |
| average | 148.3 ± 1.4 | |||||
| recommended | 148.3 ± 1.4 |
| Compound | p(⟨T⟩)/Pa | ΔcrgSm(⟨T⟩, p(⟨T⟩) | ΔcrgSm0(298.15 K, p°) | ΔcrgHm0(298.15 K) | ΔcrgGm0(298.15 K) |
|---|---|---|---|---|---|
| J·K−1·mol−1 | J·K−1·mol−1 | kJ·mol−1 | kJ·mol−1 | ||
| 5-Cl-1,10-phenanthroline | 0.0341 a | 320.3 ± 2.0 | 201.9 ± 4.3 | 113.8 ± 0.9 | 53.5 ± 2.2 |
| 0.00156 b | 344.6 ± 3.3 | 197.9 ± 5.9 | 112.2 ± 1.1 | 53.2 ± 2.9 | |
| average c | 199.9 ± 3.7 | 113.0 ± 0.7 | 53.4 ± 1.8 | ||
| 5-CH3-1,10-phenanthroline | 0.0554 a | 304.2 ± 1.9 | 190.4 ± 4.1 | 109.4 ± 0.9 | 52.6 ± 2.2 |
| 0.00247 b | 332.0 ± 2.5 | 189.4 ± 4.5 | 108.8 ± 0.9 | 52.3 ± 2.2 | |
| average c | 189.9 ± 3.0 | 109.1 ± 0.6 | 52.5 ± 1.6 | ||
| 5-CH3O-1,10-phenanthroline | 0.0446 a | 366.0± 4.0 | 252.5 ± 7.5 | 134.6 ± 1.7 | 59.3 ± 3.9 |
| 0.00287 b | 390.5 ± 4.9 | 251.5 ± 8.7 | 134.0 ± 1.8 | 59.0 ± 4.3 | |
| average c | 252.0 ± 5.7 | 134.3 ± 1.2 | 59.1 ± 2.9 | ||
| 5-CN-1,10-phenanthroline | 0.1286 a | 333.2 ± 2.4 | 230.6 ± 5.9 | 135.1 ± 1.5 | 66.4 ±3.3 |
| 0.00378 b | 359.2 ±2.6 | 224.1 ± 5.4 | 132.2 ± 1.2 | 65.4 ± 2.8 | |
| average c | 227.3 ± 4.0 | 133.7 ± 1.0 | 65.9 ± 2.2 | ||
| 5-NO2-1,10-phenanthroline | 0.3419 a | 318.6 ± 0.9 | 225.5 ± 4.8 | 133.3 ± 1.4 | 65.9 ± 2.9 |
| 0.0130 b | 352.2 ± 1.8 | 228.8 ± 4.6 | 134.2 ± 1.2 | 66.0 ± 2.5 | |
| average c | 227.3 ± 3.3 | 133.7 ± 0.8 | 66.0 ± 1.9 | ||
| 5- NH2-1,10-phenanthroline | 0.0679 a | 341.2 ± 2.3 | 234.6 ± 6.5 | 148.3 ± 1.7 | 78.3 ± 3.6 |
| 0.00322 b | 369.8 ± 4.8 | 235.4 ± 9.2 | 148.3 ± 2.1 | 78.1 ± 4.9 | |
| average c | 235.0 ± 5.6 | 148.3 ± 1.4 | 78.2 ± 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunetti, B.; Ciccioli, A.; Lapi, A.; Buzyurov, A.V.; Nagrimanov, R.N.; Varfolomeev, M.A.; Ciprioti, S.V. Sublimation Study of Six 5-Substituted-1,10-Phenanthrolines by Knudsen Effusion Mass Loss and Solution Calorimetry. Entropy 2022, 24, 192. https://0-doi-org.brum.beds.ac.uk/10.3390/e24020192
Brunetti B, Ciccioli A, Lapi A, Buzyurov AV, Nagrimanov RN, Varfolomeev MA, Ciprioti SV. Sublimation Study of Six 5-Substituted-1,10-Phenanthrolines by Knudsen Effusion Mass Loss and Solution Calorimetry. Entropy. 2022; 24(2):192. https://0-doi-org.brum.beds.ac.uk/10.3390/e24020192
Chicago/Turabian StyleBrunetti, Bruno, Andrea Ciccioli, Andrea Lapi, Aleksey V. Buzyurov, Ruslan N. Nagrimanov, Mikhail A. Varfolomeev, and Stefano Vecchio Ciprioti. 2022. "Sublimation Study of Six 5-Substituted-1,10-Phenanthrolines by Knudsen Effusion Mass Loss and Solution Calorimetry" Entropy 24, no. 2: 192. https://0-doi-org.brum.beds.ac.uk/10.3390/e24020192