Electrospun Nanofiber Membranes from 1,8-Naphthimide-Based Polymer/Poly(vinyl alcohol) for pH Fluorescence Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of PNI-SBMA
2.2.2. Preparation of Electrospun NFMs
2.2.3. Characterization
3. Results and Discussion
3.1. Design, Synthesis, and Characterization of PNI-SBMA
3.2. Preparation and Characterization of NFMs
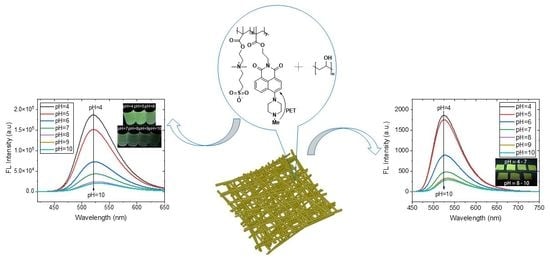
3.3. pH Fluorescence Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Banna, M.H.; Najjaran, H.; Sadiq, R.; Imran, S.A.; Rodriguez, M.J.; Hoorfar, M. Miniaturized water quality monitoring pH and conductivity sensors. Sens. Actuators B Chem. 2014, 193, 434–441. [Google Scholar] [CrossRef]
- Cao, L.; Sun, G.; Zhang, C.; Liu, W.; Li, J.; Wang, L. An Intelligent Film Based on Cassia Gum Containing Bromothymol Blue-Anchored Cellulose Fibers for Real-Time Detection of Meat Freshness. J. Agric. Food Chem. 2019, 67, 2066–2074. [Google Scholar] [CrossRef]
- Venkatesan, M.; Veeramuthu, L.; Liang, F.-C.; Chen, W.-C.; Cho, C.-J.; Chen, C.-W.; Chen, J.-Y.; Yan, Y.; Chang, S.-H.; Kuo, C.-C. Evolution of electrospun nanofibers fluorescent and colorimetric sensors for environmental toxicants, pH, temperature, and cancer cells—A review with insights on applications. Chem. Eng. J. 2020, 397, 125431. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yan, B. An efficient and sensitive fluorescent pH sensor based on amino functional metal-organic frameworks in aqueous environment. Dalton Trans 2016, 45, 7078–7084. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Lu, K.; Lin, W. Nanoscale metal-organic frameworks for real-time intracellular pH sensing in live cells. J. Am. Chem. Soc. 2014, 136, 12253–12256. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, L.; Ma, F.; Liu, W.; Wang, Y.; Silver, M.A.; Chen, L.; Zhu, L.; Gui, D.; Diwu, J.; et al. Covalent Organic Framework Functionalized with 8-Hydroxyquinoline as a Dual-Mode Fluorescent and Colorimetric pH Sensor. ACS Appl. Mater. Interfaces 2018, 10, 15364–15368. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Kwon, H.-J.; Howlader, M.M.R.; Deen, M.J. Microfabricated electrochemical pH and free chlorine sensors for water quality monitoring: Recent advances and research challenges. RSC Adv. 2015, 5, 69086–69109. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, X.; Boezen, H.M.; Huo, X. Children with health impairments by heavy metals in an e-waste recycling area. Chemosphere 2016, 148, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Weihe, P.; White, R.F.; Debes, F. Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environ. Res. 1998, 77, 165–172. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef]
- Agarwal, A.; Raheja, A.; Natarajan, T.S.; Chandra, T.S. Development of universal pH sensing electrospun nanofibers. Sens. Actuators B Chem. 2012, 161, 1097–1101. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Abdul Hamid, M.A.; Ahmed, N.M.; Jalar, A.; Shamsudin, R.; Othman, N.K.; Kar Keng, L.; Chiu, W.; Al-Rawi, H.N. High Sensitivity pH Sensor Based on Porous Silicon (PSi) Extended Gate Field-Effect Transistor. Sensors 2016, 16, 839. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Bonefacino, J.; Guan, B.O.; Tam, H.Y. All-polymer fiber-optic pH sensor. Opt. Express 2018, 26, 14610–14616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Gan, J.; Chen, K.; He, J.; Song, Q.L.; Hou, X.Y. Positive and negative fluorescent imaging induced by naphthalimide polymers. J. Mater. Chem. 2002, 12, 1262–1267. [Google Scholar] [CrossRef]
- Gotor, R.; Ashokkumar, P.; Hecht, M.; Keil, K.; Rurack, K. Optical pH Sensor Covering the Range from pH 0–14 Compatible with Mobile-Device Readout and Based on a Set of Rationally Designed Indicator Dyes. Anal. Chem. 2017, 89, 8437–8444. [Google Scholar] [CrossRef] [Green Version]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Y.; Cao, H.; Duan, F.; Su, C.; Lu, C.; Chang, J.; Ding, H. High-selectivity membrane absorption process for recovery of ammonia with electrospun hollow fiber membrane. Sep. Purif. Technol. 2019, 216, 136–146. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Song, J.; Yang, J.; Du, Z.; Zhao, W.; Guo, H.; Wen, C.; Li, Q.; Sui, X.; et al. A Multifunctional Pro-Healing Zwitterionic Hydrogel for Simultaneous Optical Monitoring of pH and Glucose in Diabetic Wound Treatment. Adv. Funct. Mater. 2019, 30, 1905493. [Google Scholar] [CrossRef]
- Wei, Z.; Zhou, Z.-K.; Li, Q.; Xue, J.; Di Falco, A.; Yang, Z.; Zhou, J.; Wang, X. Flexible nanowire cluster as a wearable colorimetric humidity sensor. Small 2017, 13, 1700109. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, H.; Yang, M. TiO2 nanoparticles supported on PMMA nanofibers for photocatalytic degradation of methyl orange. J. Colloid Interface Sci. 2017, 508, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Razali, R.A.; Lokanathan, Y.; Chowdhury, S.R.; Saim, A.; Idrus, R.H. Physicochemical and structural characterization of surface modified electrospun PMMA nanofiber. Sains Malays. 2018, 47, 1787–1794. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Q.; Sun, L.; Wang, H.; Zhang, C.; Fei, X.; Sun, M.; Li, Y. Preparation of fluorescent nanofibrous film as a sensing material and adsorbent for Cu2+ in aqueous solution via copolymerization and electrospinning. J. Hazard. Mater. 2011, 194, 185–192. [Google Scholar] [CrossRef]
- Piloto, C.; Mirri, F.; Bengio, E.A.; Notarianni, M.; Gupta, B.; Shafiei, M.; Pasquali, M.; Motta, N. Room temperature gas sensing properties of ultrathin carbon nanotube films by surfactant-free dip coating. Sens. Actuators B Chem. 2016, 227, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Paulo, S.; Palomares, E.; Martinez-Ferrero, E. Graphene and Carbon Quantum Dot-Based Materials in Photovoltaic Devices: From Synthesis to Applications. Nanomaterials 2016, 6, 157. [Google Scholar] [CrossRef]
- Schoolaert, E.; Steyaert, I.; Vancoillie, G.; Geltmeyer, J.; Lava, K.; Hoogenboom, R.; De Clerck, K. Blend electrospinning of dye-functionalized chitosan and poly(epsilon-caprolactone): Towards biocompatible pH-sensors. J. Mater. Chem. B 2016, 4, 4507–4516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, Z.; Bai, L.; Liu, Y. Nitrogen-doped carbon dots derived from electrospun carbon nanofibers for Cu(ii) ion sensing. New J. Chem. 2019, 43, 1812–1817. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Sensing Behavior to ppm-Level Gases and Synergistic Sensing Mechanism in Metal-Functionalized RGO-Loaded Zno Nanofibers. Sens. Actuators B Chem. 2018, 255, 1884–1896. [Google Scholar] [CrossRef]
- Yu, X.; Liu, W.; Deng, X.; Yan, S.; Su, Z. Gold Nanocluster Embedded Bovine Serum Albumin Nanofibers-Graphene Hybrid Membranes for the Efficient Detection and Separation of Mercury Ion. Chem. Eng. J. 2018, 335, 176–184. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T. Design of WO3-SnO2 Core-Shell Nanofibers and their Enhanced Gas Sensing Performance Based on Different Work Function. Appl. Surf. Sci. 2018, 442, 30–37. [Google Scholar] [CrossRef]
- Tahvili, A.; Poush, M.k.; Ahmed, M.; Parsaee, Z. New Efficient Inorganic-Organic Nanofibers Electrospun Membrane for Fluorescence Detection and Removal of Mercury (II) Ions. J. Mol. Struct. 2019, 1179, 242–251. [Google Scholar] [CrossRef]
- Zhao, L.; Xie, S.; Song, X.; Wei, J.; Zhang, Z.; Li, X. Ratiometric fluorescent response of electrospun fibrous strips for real-time sensing of alkaline phosphatase in serum. Biosens. Bioelectron. 2017, 91, 217–224. [Google Scholar] [CrossRef]
- Pan, N.; Qin, J.; Feng, P.; Li, Z.; Song, B. Color-changing smart fibrous materials for naked eye real-time monitoring of wound pH. J. Mater. Chem. B 2019, 7, 2626–2633. [Google Scholar] [CrossRef]
- Chen, B.Y.; Kuo, C.C.; Huang, Y.S.; Lu, S.T.; Liang, F.C.; Jiang, D.H. Novel highly selective and reversible chemosensors based on dualratiometric fluorescent electrospun nanofibers with pH and Fe3+ modulated multicolor fluorescence emission. ACS Appl. Mater. Interfaces 2015, 7, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-Y.; Wu, W.-X.; Wang, N.; Yu, X.-Q. Novel biocompatible fluorescent polymeric micelles based on 1,8-naphthalimide derivatives for cell imaging. Polym. Chem. 2015, 6, 364–368. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Bojinov, V.B.; Nikolov, P.S. The design, synthesis and photophysical properties of two novel 1,8-naphthalimide fluorescent pH sensors based on PET and ICT. Dyes Pigments 2011, 88, 350–357. [Google Scholar] [CrossRef]
- Du, W.; Xu, J.; Li, H.; Feng, C.; Yu, M.; Li, Z.; Wei, L. Naked-eye and fluorescence detection of basic pH and F− with a 1,8-naphthalimide-based multifunctional probe. RSC Adv. 2015, 5, 15077–15083. [Google Scholar] [CrossRef]
- Wang, W.; Wen, Q.; Zhang, Y.; Fei, X.; Li, Y.; Yang, Q.; Xu, X. Simple naphthalimide-Based fluorescent sensor for highly sensitive and selective detection of Cd2+ and Cu2+ in aqueous solution and living cells. Dalton Trans. 2013, 42, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Zhu, W.; Meng, X.; Guo, Z.; Tian, H. A hydrophilic fluorescent polymer containing naphthalimide moiety as chemosensor for microbioreactors. Sci. China Ser. B Chem. 2009, 52, 821–826. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, L.; Li, Q.; Li, J.; Zhu, X.; Jiang, Y.; Sha, O.; Yang, X.; Xin, J.H.; Wang, J.; et al. Durable Antibacterial and Nonfouling Cotton Textiles with Enhanced Comfort via Zwitterionic Sulfopropylbetaine Coating. Small 2016, 12, 3516–3521. [Google Scholar] [CrossRef]
- Yarin, A.L.; Kataphinan, W.; Reneker, D.H. Branching in electrospinning of nanofibers. J. Appl. Phys. 2005, 98, 064501. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Huang, X.; Wu, B. Some fascinating phenomena in electrospinning processes and applications of electrospun nanofibers. Polym. Int. 2007, 56, 1330–1339. [Google Scholar] [CrossRef]
- Chen-Ming, H.; Shivkumar, S. Nano-sized beads and porousfiber constructs of poly(ε-caprolactone) produced by electrospinning. J. Mater. Sci. 2004, 39, 3003–3013. [Google Scholar]
- Ismail, H.M.; Zamani, S.; Elrayess, M.A.; Kafienah, W.; Younes, H.M. New Three-Dimensional Poly(decanediol-co-tricarballylate) Elastomeric Fibrous Mesh Fabricated by Photoreactive Electrospinning for Cardiac Tissue Engineering Applications. Polymers 2018, 10, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Su, F.; Weber, W.; Nandakumar, V.; Shumway, B.R.; Jin, Y.; Zhou, X.; Holl, M.R.; Johnson, R.H.; Meldrum, D.R. A series of naphthalimide derivatives as intra and extracellular pH sensors. Biomaterials 2010, 31, 7411–7422. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.G.; Zeng, G.M.; Chen, L.X.; Shen, G.L.; Yu, R.Q. Proton “off-on” behaviour of methylpiperazinyl derivative of naphthalimide: A pH sensor based on fluorescence enhancement. Analyst 2004, 129, 20–24. [Google Scholar] [CrossRef]
- Niu, C.G.; Gui, X.Q.; Zeng, G.M.; Yuan, X.Z. A ratiometric fluorescence sensor with broad dynamic range based on two pH-sensitive fluorophores. Analyst 2005, 130, 1551–1556. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Liu, X.; Jia, J.; Wu, H.; Xie, J.; Jia, Y. Electrospun Nanofiber Membranes from 1,8-Naphthimide-Based Polymer/Poly(vinyl alcohol) for pH Fluorescence Sensing. Molecules 2022, 27, 520. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27020520
Xu L, Liu X, Jia J, Wu H, Xie J, Jia Y. Electrospun Nanofiber Membranes from 1,8-Naphthimide-Based Polymer/Poly(vinyl alcohol) for pH Fluorescence Sensing. Molecules. 2022; 27(2):520. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27020520
Chicago/Turabian StyleXu, Le, Xi Liu, Jiao Jia, Hao Wu, Juan Xie, and Yongtang Jia. 2022. "Electrospun Nanofiber Membranes from 1,8-Naphthimide-Based Polymer/Poly(vinyl alcohol) for pH Fluorescence Sensing" Molecules 27, no. 2: 520. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27020520