Mononuclear Transition Metal Cymantrenecarboxylates as Precursors for Spinel-Type Manganites
Abstract
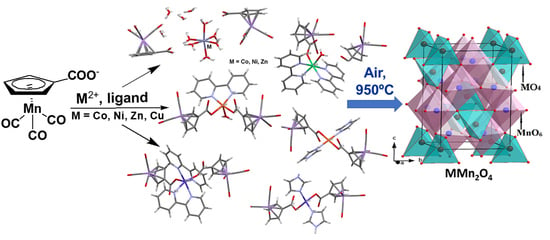
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure of 1–8
2.2. Magnetic Properties of Co Complexes 1, 4, and 5
2.3. Solid-State Thermolysis of Complexes 1–8 in Air Atmosphere
3. Materials and Methods
3.1. Synthesis of [M(H2O)6](CymCO2)2·4H2O, (M = Co, Ni, Zn; 1–3)
3.2. Synthesis of [Co(CymCO2)2(imz)2] (4), [Co(CymCO2)2(bpy)2]·2PhMe (5), [Ni(CymCO2)(bpy)2(H2O)][CymCO2]·0.5MePh·2H2O (6), and [Cu(CymCO2)2(bpy)(H2O)] (8)
3.3. Synthesis of [Cu(CymCO2)2(imz)2] (7)
3.4. X-ray Data Collection
3.5. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Irving, H.M.N.H.; Tomlinson, W.R. Complexes of hydroxy-acids containing two different metals. Chem. Commun. 1968, 9, 497. [Google Scholar] [CrossRef]
- Sinn, E.; Harris, C.M. Schiff base metal complexes as ligands. Coord. Chem. Rev. 1969, 4, 391–422. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Gao, W.-X.; Lin, L.; Jin, G.-X. Recent advances in the construction and applications of heterometallic macrocycles and cages. Coord. Chem. Rev. 2017, 344, 323–344. [Google Scholar] [CrossRef]
- Langley, S.K.; Chilton, N.F.; Moubarakia, B.; Murray, K.S. Structure and magnetic exchange in heterometallic 3d-3d transition metal triethanolamine clusters. Dalton Trans. 2012, 41, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; Martí-Gastaldo, C.; Romero, F.M. Multifunctionality in hybrid magnetic materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011, 40, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Kokozay, V.N.; Vassilyeva, O.Y. Direct synthesis of heterometallic complexes. Trans. Met. Chem. 2002, 27, 693–699. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Novotortsev, V.M. Heterometallic Carboxylate Complexes as Precursors for Mixed Oxides: II. d–d Carboxylates. Russ. J. Gen. Chem. 2018, 88, 1290–1305. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Novotortsev, V.M. Heterometallic Carboxylate Complexes as Precursors for Mixed Oxides: III. 3d–4f Carboxylates. Russ. J. Gen. Chem. 2018, 88, 1306–1317. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Ilyukhin, A.B.; Belova, E.V.; Yapryntsev, A.D.; Khrushcheva, A.V.; Loktev, A.S. New simple La-Ni complexes as efficient precursors for functional LaNiO3-based ceramics. Appl. Organomet. Chem. 2021, 36, e6519. [Google Scholar] [CrossRef]
- Hou, H.; Li, L. Progress in ferrocene carboxylate metal complexes. In Leading Edge Organometallic Chemistry Research; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2006; pp. 27–74. [Google Scholar]
- Zhang, E.; Hou, H.; Meng, X.; Liu, Y.; Liu, Y.; Fan, Y. Ferrocenyl Functional Coordination Polymers Based on Mono-, Bi-, and Heterotrinuclear Organometallic Building Blocks: Syntheses, Structures, and Properties. Cryst. Growth Des. 2009, 9, 903–913. [Google Scholar] [CrossRef]
- Pasynskii, A.A.; Shapovalov, S.S.; Gordienko, A.V.; Razuvaev, D.I.; Skabitsky, I.V.; Aleksandrov, G.G.; Dobrohotova, Z.W.; Bogomyakov, A.S. Dimeric “paddle-wheel” cymantrenylcarboxylates of copper (II). Inorg. Chim. Acta 2012, 384, 18–22. [Google Scholar] [CrossRef]
- Pasynskii, A.A.; Shapovalov, S.S.; Gordienko, A.V.; Skabitskii, I.V. Cymantrenecarboxylate complexes of nickel(II) and cobalt(II). Russ. J. Coord. Chem. 2011, 37, 127–132. [Google Scholar] [CrossRef]
- Shapovalov, S.S.; Pasynskii, A.A.; Skabitskii, I.V.; Krishtop, T.A.; Dobrokhotova, Z.V. Synthesis and molecular structures of cymantrenecarboxylate derivatives of titanium(IV) and vanadium(III) cyclopentadienyl complexes and of copper(II) and manganese(II) lutidine complexes. Russ. J. Coord. Chem. 2014, 40, 77–83. [Google Scholar] [CrossRef]
- Uvarova, M.A.; Nefedov, S.E. Transformations of Polymers of 4,4′-Dipyridyl and Cobalt(II) and Manganese(II) Cymantrenates in the Presence of N-Donors of Different Denticity. Russ. J. Inorg. Chem. 2021, 66, 1660–1668, and references therein. [Google Scholar] [CrossRef]
- Kaye, S.S.; Long, J.R. Matrix Isolation Chemistry in a Porous Metal−Organic Framework: Photochemical Substitutions of N2 and H2 in Zn4O[(η6-1,4-Benzenedicarboxylate)Cr(CO)3]3. J. Am. Chem. Soc. 2008, 130, 806–807. [Google Scholar] [CrossRef] [PubMed]
- Murugesapandian, B.; Roesky, P.W. Coordination Polymers of Zinc with (η6-Benzenecarboxylate) Chromium Tricarbonyl. Inorg. Chem. 2011, 50, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Gau, H.-M.; Chen, C.-T.; Jong, T.-T.; Chien, M.-Y. Group IV metal-chromium complexes bridged by a benzoate group. J. Organomet. Chem. 1993, 448, 99–106. [Google Scholar] [CrossRef]
- Kettner, F.; Kischel, M.; Krautscheid, H. Coordination polymers based on 1,1′-cobaltocenium dicarboxylate linkers. CrystEngComm. 2013, 15, 8437–8443. [Google Scholar] [CrossRef]
- Kondo, M.; Hayakawa, Y.; Miyazawa, M.; Oyama, A.; Unoura, K.; Kawaguchi, H.; Naito, T.; Maeda, K.; Uchida, F. A New Redox-Active Coordination Polymer with Cobalticinium Dicarboxylate. Inorg. Chem. 2004, 43, 5801–5803. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Gavrikov, A.V.; Novotortsev, V.M. Specific features of the structure, reactivity, thermolysis, and magnetism of cymantrenecarboxylate complexes of lanthanides. Russ. J. Coord. Chem. 2016, 42, 591–603. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Ilyukhin, A.B.; Babeshkin, K.A.; Belova, E.V.; Gavrikov, A.V.; Efimov, N.N. Linear Tetranuclear Lanthanide Cymantrenecarboxylates with Diethylene Glycol Ligand: Synthesis, Magnetism, and Thermolysis. Eur. J. Inorg. Chem. 2021, 2, 147–155, and references therein. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Efimov, N.N.; Ilyukhin, A.B.; Dobrokhotova, Z.V.; Novotortsev, V.M. Tetranuclear LnIII2MnII2 cymantrenecarboxylates. Synthesis, structure, thermolysis and magnetic properties. Inorg. Chim. Acta 2014, 418, 157–162. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Efimov, N.N.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Gavrikov, A.V.; Novotortsev, V.M. Binuclear and polynuclear cymantrenecarboxylate complexes of heavy lanthanides. Russ. J. Coord. Chem. 2015, 41, 149–161. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Ilyukhin, A.B.; Koroteev, P.S. Step-by-step: Uncommon SCSC transformation accompanied by stepwise change in the binding of a particular ligand within a mononuclear complex upon stepwise desolvation. CrystEngComm. 2020, 22, 2895–2899. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Belova, E.V.; Ilyukhin, A.B.; Koroteev, P.S.; Sadovnikov, A.A. Preparation and properties of uncommon Cd-Mn carboxylate complexes—per se and as precursors for CdMn2O4-based ceramics. Appl. Organomet. Chem. 2021, 35, e6190. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Gavrikov, A.V.; Novotortsev, V.M. Polymer lanthanide cymantrenecarboxylates. Russ. J. Coord. Chem. 2015, 41, 805–816. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Kirdyankin, D.I.; Tyurin, A.V.; Gavrikov, A.V.; Novotortsev, V.M. Polymeric lanthanide acetates with peripheral cymantrenecarboxylate groups—Synthesis, magnetism and thermolysis. Polyhedron 2015, 85, 941–952. [Google Scholar] [CrossRef]
- Kannan, S.; Venkatachalam, G.; Lee, H.-J.; Min, B.K.; Kim, W.; Koo, E.; Do, Y.R.; Yoon, S. Mononuclear transition metal complexes with sterically hindered carboxylate ligands: Synthesis, structural and spectral properties. Polyhedron 2011, 30, 340–346. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Grineva, A.A.; Uvarova, M.A.; Datchuk, R.R.; Nefedov, S.E. Effects of the Nature of Transition Metal on the Composition and Structure of Reaction Products of M[(OOCC5H4)Mn(CO)3]2[O(H)Me]4 (M = Cu(II), Ni(II), Co(II), or Mn(II)) with 1,10-Phenanthroline. Rus. J. Inorg. Chem. 2018, 63, 610–617. [Google Scholar] [CrossRef]
- Tripathi, S.; Dey, A.; Shanmugam, M.; Narayanan, R.S.; Chandrsekhar, V. Cobalt(II) Complexes as Single-Ion Magnets. In Organometallic Magnets; Springer: Cham, Germany, 2018; pp. 35–75. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Long, J.R. Slow Magnetic Relaxation at Zero Field in the Tetrahedral Complex [Co(SPh)4]2−. J. Am. Chem. Soc. 2011, 133, 20732–20734. [Google Scholar] [CrossRef]
- Świtlicka, A.; Machura, B.; Penkala, M.; Bieńko, A.; Bieńko, D.C.; Titiš, J.; Rajnák, C.; Boča, R.; Ozarowski, A.; Ozerov, M. Slow Magnetic Relaxation in Cobalt(II) Field-Induced Single-Ion Magnets with Positive Large Anisotropy. Inorg. Chem. 2018, 57, 12740–12755. [Google Scholar] [CrossRef]
- Leuenberger, M.N.; Loss, D.J.N. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardavan, A.; Rival, O. Will spin-relaxation times in molecular magnets permit quantum information processing? Phys. Rev. Lett. 2007, 98, 057201. [Google Scholar] [CrossRef] [Green Version]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.V.; Pavlov, A.A.; Nelyubina, Y.V.; Boulon, M.-E.; Varzatskii, O.A.; Voloshin, Y.Z.; Winpenny, R.E.P. A Trigonal Prismatic Mononuclear Cobalt(II) Complex Showing Single-Molecule Magnet Behavior. J. Am. Chem. Soc. 2015, 137, 9792–9795. [Google Scholar] [CrossRef]
- Rechkemmer, Y.; Breitgoff, F.D.; van der Meer, M.; Atanasov, M.; Hakl, M.; Orlita, M.; Neugebauer, P.; Neese, F.; Sarkar, B.; van Slageren, J. A four-coordinate cobalt(II) single-ion magnet with coercivity and a very high energy barrier. Nat. Commun. 2016, 7, 10467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankratova, Y.A.; Nelyubina, Y.V.; Novikov, V.V.; Pavlov, A.A. High-Spin Cobalt(II) Complex with Record-Breaking Anisotropy of the Magnetic Susceptibility According to Paramagnetic NMR Spectroscopy Data. Russ. J. Coord. Chem. 2021, 47, 10–16. [Google Scholar] [CrossRef]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Saunders College Publishing: Philadelphia, PA, USA, 1976; p. 658. [Google Scholar]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. Phi: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear D- and F-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Carlin, R.L. Magnetochemistry; Springer: Berlin, Germany, 1986; p. 65. [Google Scholar]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics, I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Kirdyankin, D.I.; Tyurin, A.V.; Velikodny, Y.A.; Kovba, M.L.; Novotortsev, V.M. Lanthanide cymantrenecarboxylate complexes with an Ln:Mn ratio of 1:2 as precursors for LnMn2O5 phases. Synthesis, structure, physicochemical properties, and thermal decomposition. Polyhedron 2013, 65, 110–121. [Google Scholar] [CrossRef]
- Nelson-Cheeseman, B.B.; Chopdekar, R.V.; Iwata, J.M.; Toney, M.F.; Arenholz, E.; Suzuki, Y. Modified magnetic ground state in NiMn2O4 thin films. Phys. Rev. B 2010, 82, 144419. [Google Scholar] [CrossRef] [Green Version]
- Kutty, R.K.N.; Kasturi, P.R.; Jaganath, J.; Padmanapan, S.; Lee, Y.S.; Meyrick, D.; Selvan, R.K. Structural and magnetic properties of CoMn2O4 synthesized by auto combustion method. J. Mater. Sci: Mater. Electron. 2019, 30, 975–981. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kurokawa, A.; Takeuchi, H.; Yano, S.; Yanoh, T.; Onuma, K.; Kondo, T.; Miike, K.; Ichiyanagi, Y. Magnetic Properties and X-ray Absorption Fine-Structure Spectra of CoMn2O4 Nanoparticles. e-J. Surf. Sci. Nanotechnol. 2012, 10, 643–646. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Sunlight Induced Photocatalytic Degradation of Organic Pollutants by Biosynthesized Heterometallic Oxides Nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 61760–61780. [Google Scholar] [CrossRef]
- Lin, C.; Shi, D.; Wu, Z.; Zhang, L.; Zhai, Z.; Fang, Y.; Sun, P.; Han, R.; Wu, J.; Liu, H. CoMn2O4 Catalyst Prepared Using the Sol-Gel Method for the Activation of Peroxymonosulfate and Degradation of UV Filter 2-Phenylbenzimidazole- 5-sulfonic Acid (PBSA). Nanomaterials 2019, 9, 774. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, X.; Zhao, Q.; Liu, S. Synthesis and optical property of one-dimensional spinel ZnMn2O4 nanorods. Nanoscale Res. Lett. 2011, 6, 323. [Google Scholar] [CrossRef] [Green Version]
- Mani, M.P.; Venkatachalam, V.; Thamizharasan, K.; Jothibas, M. Evaluation of Cubic-Like Advanced ZnMn2O4 Electrode for High-Performance Supercapacitor Applications. J. Electron. Mater. 2021, 50, 4381–4387. [Google Scholar] [CrossRef]
- Pattanayak, B.; Simanjuntak, F.M.; Panda, D.; Yang, C.C.; Kumar, A.; Le, P.A.; Wei, K.H.; Tseng, T.Y. Role of precursors mixing sequence on the properties of CoMn2O4 cathode materials and their application in pseudocapacitor. Sci. Rep. 2019, 9, 16852. [Google Scholar] [CrossRef]
- Csete de Györgyfalva, G.D.C.; Nolte, A.N.; Reaney, I.M. Correlation between microstructure and conductance in NTC thermistors produced from oxide powders. J. Eur. Ceram. Soc. 1999, 19, 857–860. [Google Scholar] [CrossRef]
- Tomaszewicz, E.; Kotfica, M. Mechanism and kinetics of thermal decomposition of nickel(II) sulfate(VI) hexahydrate. J. Therm. Anal. Calorim. 2004, 77, 25–31. [Google Scholar] [CrossRef]
- Netskina, O.; Mucha, S.; Veselovskaya, J.; Bolotov, V.; Komov, O.; Ishchenko, A.; Bulavchenko, O.; Prosvirin, I.; Pochtar, A.; Rogov, V. CO2 Methanation: Nickel–Alumina Catalyst Prepared by Solid-State Combustion. Materials 2021, 14, 6789. [Google Scholar] [CrossRef]
- Parveen, N.; Nazir, R.; Mazhar, M. Thermal degradation pathways of nickel(II) bipyridine complexes to size-controlled nickel nanoparticles. J. Therm. Anal. Calorim. 2013, 111, 93–99. [Google Scholar] [CrossRef]
- Dhar, S.K.; Basolo, F. Thermal decomposition of the tris (2,2′-bipyridine) complexes of some first row transition group elements in the solid state. J. Inorg. Nucl. Chem. 1963, 25, 37–44. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Anisimov, K.N.; Kolobova, N.E.; Makarov, Y.V. Metalation of cyclopentadienyltricarbonylmanganese. Russ. Chem. Bull. 1968, 17, 672. [Google Scholar] [CrossRef]
- Bruker. APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2007. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]

















| Parameter | 1 | 4 | 5 |
|---|---|---|---|
| gx | 2.6 ± 0.8 | 2.3 ± 0.1 | 2.98 ± 0.2 |
| gy | 2.1 ± 0.9 | 1.9 ± 0.1 | 2.3 ± 0.3 |
| gz | 2.657 ± 0.006 | 2.29 ± 0.01 | 2.777 ± 0.002 |
| D, cm−1 | −75 ± 2 | −14.5 ± 0.6 | −65.5 ± 0.5 |
| TIP, cm−1 | - | 8 × 10−4 (fixed) | - |
| Residual, % | 99.689 | 99.666 | 99.986 |
| Complex | 1 | 4 | 5 |
|---|---|---|---|
| Field, Oe | 2500 | 1500 | 1000 |
| Temperature range, K | 7–7.2 | 2–3 | 3.5–4.5 |
| ΔE/kB, K | 44 | 13 | 10 |
| τ0, s | 4 × 10−8 | 3 × 10−7 | 3.0 × 10−6 |
| Temperature range, K | 2–7.2 | 2–3 | 2–4.5 |
| C, K−nRaman·s−1 | 3.5 | 135 | 336 |
| nRaman | 4.7 | 5.4 | 3.04 |
| A, K−1Oe−4s−1 | 9.9 × 10−12 | - | 1.91 × 10−9 |
| R2 | 0.99937 | 0.99888 | 0.99994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koroteev, P.S.; Ilyukhin, A.B.; Gavrikov, A.V.; Babeshkin, K.A.; Efimov, N.N. Mononuclear Transition Metal Cymantrenecarboxylates as Precursors for Spinel-Type Manganites. Molecules 2022, 27, 1082. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27031082
Koroteev PS, Ilyukhin AB, Gavrikov AV, Babeshkin KA, Efimov NN. Mononuclear Transition Metal Cymantrenecarboxylates as Precursors for Spinel-Type Manganites. Molecules. 2022; 27(3):1082. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27031082
Chicago/Turabian StyleKoroteev, Pavel S., Andrey B. Ilyukhin, Andrey V. Gavrikov, Konstantin A. Babeshkin, and Nikolay N. Efimov. 2022. "Mononuclear Transition Metal Cymantrenecarboxylates as Precursors for Spinel-Type Manganites" Molecules 27, no. 3: 1082. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27031082