Developing Potential Candidates of Preclinical Preeclampsia
Abstract
:1. Introduction
2. Results
2.1. LC-MS/MS MRM Assays
2.1.1. Literature and Proteome Database Search
| Gene Symbol_Dysregulation in Preeclampsia [7] * | Protein Name_UniProt Accession Number; Protein Expression in Normal Tissue | Blood_Concentration | ABs_Kits † |
|---|---|---|---|
| CCK ↑ | Cholecystokinin_P06307; Most normal tissues weak to moderate, gastrointestinal tract (GI) and a subset of neuronal cells strongly stained. Trophoblast (TB) moderate/Decidua negative (neg) [15] | 1.3–4.2 pmol/L, 2nd trimester [16] 8.7 ± 1.2, 10.1 ± 1.6 and 10.4 ± 1.2 pM in 1st, 2nd, 3rd trimesters [17] | 59_6 |
| C4orf10 13.8 ↑ | ---** | - | - |
| S100A8 ↑ | Protein S100-A8_P05109; Selective nuclear and cytoplasmic expression in squamous epithelia, subsets of cells outside reaction centra of lymphoid tissues and subsets of bone marrow poietic cells. TB neg/decidua neg [15] | 2.7 × 105 pg/mL Plasma [18] | 33_6 |
| CTAG2 ↑ | Isoform LAGE-1B of Cancer/testis antigen 2 (LAGE-1L, Isoform LAGE-1A of Cancer/testis antigen 2 [LAGE-1S])_O75638-1, O75638-2; Peripheral blood mononuclear leukocytes [19]. Pending in The Human Protein Atlas (THPA) [15] | PBMCs [19] | 8_0 |
| CDNA: FLJ22732 8.4 ↑ | ---** | - | - |
| MUC15 -8.0 ↓ | Isoform 1 of Mucin-15 (Cell membrane; Single-pass type I membrane protein), Isoform 2 of Mucin-15 (Secreted)_Q8N387-1, Q8N387-2; Normal tissues in general neg. Hepatocytes strong positivity. Strong staining in subset of cells in seminiferous ducts of testis. TB moderate/Decidua neg [15] | - | 5_0 |
| LOC440157 -8.1 ↓ | Full-length cDNA 5-PRIME end of clone CS0DK007YB08 of HeLa cells of Homo sapiens (UniProt)_Q6NYL1 | - | - |
| FN1 -8.2 ↓ | Fibronectin (Isoforms 1 to 15)_P02751−1 to −15; Extracellular matrix, stromal cells, serum, bone marrow poetic cells and placenta show moderate to strong positivity. TB & Decidua moderate [15] | 1.40 × 106 pg/mL Plasma [18,20,21]; 11–43 μg/mL term gestational hypertension, PE, controls [21] | 78_5 |
| OXGR1 -8.2 ↓ | 2-Oxoglutarate receptor 1_Q96P68; Myometrium [19]. Pending THPA [15] | - | 2_1 |
| ELL2 -8.2 ↓ | RNA polymerase II elongation factor ELL2_O00472; Most normal tissues moderate to strong positivity. Distinct membranous positivity in prostate, pancreatic islets, renal tubules. Intestine, bile ducts, cells in CNS, heart and skeletal muscle stained weak or neg. TB strong/Dedicua moderate [15] | - | 8_0 |
| SCARA5 -8.3 ↓ | Scavenger receptor class A member 5_Q6ZMJ2-1; Most normal tissues moderate positivity. Strong in GI tract, gall bladder, adrenal gland and lymphoid cells outside the reaction center. Hepatocytes, pancreas, CNS, respiratory and squamous epithelia weak or neg. TB & Decidua weak [15] | - | 6_0 |
| SLC16A6 -8.4 ↓ | Monocarboxylate transporter 7_O15403; T lymphocytes, monocytes, plasma, tonsil [19]. TB neg; decidua not available [15] | Plasma [18] | 1_1 |
| SEMA3C -8.4 ↓ | Semaphorin-3C_Q99985; Peripheral blood, adipose tissue, dendritic cells, endometrium, epithelial cell lines, fibroblasts, monocytes, neutrophils, ovarian surface epithelium, plasma, skin cell lines, stromal cells, uterus [19]. TB & decidua neg [15] | Blood [19] | 6_0 |
| F11R -8.4 ↓ | Junctional adhesion molecule A_Q9Y624; Moderate to strong cytoplasmic staining in majority of normal tissues. TB & Decidua strong [15] | Plasma (peptideatlas) Platelets, RBCs [22] T lymphocytes [23]; 74.1–293.2 pg/mL [24] | 10_1 |
| CFH /// CFHR1 -8.4 ↓ | Complement factor H (Isoforms 1 to 2) Complement factor H-related protein 1_Q03591; Strong expression in connective tissue and plasma. TB & Decidua neg [15] | Plasma [18] 0.5 mg/mL in serum [25] | 52_3 |
| CDNA clone IMAGE:5267797 -8.6 ↓ | Interleukin-1 receptor-associated kinase 4_Q9NWZ3; Moderate in many squamous tissues, strong in glandular tissue types. TB strong/Decidua moderate [15] | Plasma [18] | 0_1 |
| SLCO4A1 -8.6 ↓ | Solute carrier organic anion transporter family member 4A1 (Isoforms 1 to 4)_Q96BD0-1; Granular cytoplasmic expression in selected tissues. TB moderate-weak/Decidua neg [15] | - | - |
| KRT14 -8.7 ↓ | Keratin, type I cytoskeletal 14_P02533; Myoepithelial cells, squamous epithelium. TB & Decidua moderate [15] | Plasma [18] | 11_1 |
| MAGEB6 -8.9 ↓ | Melanoma-associated antigen B6_Q8N7X4; TB strong/Decidua not available [15] | - | 6_0 |
| TNRC9 -9.0 ↓ | TOX high mobility group box family member 3 (CAG trinucleotide repeat-containing gene F9 protein, Trinucleotide repeat-containing gene 9 protein)_O15405; TB & Decidua neg [15] | - | 5_0 |
| TMC4 -9.0 ↓ | Transmembrane channel-like protein 4 (Isoforms 1 to 3)_Q7Z404−1 to −3; TB & Decidua neg [15] | - | 3_0 |
| ASCL2 -9.2 ↓ | Achaete-scute homolog 2_Q99929 ; Cytotrophoblastic cells, T lymphocytes, placenta, tonsil [19]. Pending THPA [15] | - | - |
| DEPDC7 -9.4 ↓ | DEP domain-containing protein 7 (Protein TR2/D15 Isoforms 1 to 2)_Q96QD5−1 to −2; Most normal tissues strong positivity. TB & Decidua strong [15] | - | 5_0 |
| RUFY3 -9.6 ↓ | Protein RUFY3 (Rap2-interacting protein x, Isoforms 1 to 2)_Q7L099−1 to −2; T lymphocytes, tonsil [19]. TB & Decidua moderate [15] | - | 4_0 |
| CDNA clone IMAGE:5287025 -9.7 ↓ | Cytochrome c oxidase subunit I_D9YT98; Retina, liver, brain, neurons, renal cortex, amniotic fluid, endothelial cells, high endothelial postcapillary venule, macrophages, placenta, prostate gland, tonsil [19] | Other subunits in plasma [18] | 2_0 |
| HPS3 -9.8 ↓ | Hermansky-Pudlak syndrome 3 protein (Isoforms 1 and 2)_Q969F9-1; Brain, fibroblasts, heart, kidney, liver, lung, pancreatic, placental, skeletal [19]. TB moderate/Decidua strong [15] | - | 13_0 |
| LRAP -9.9 ↓ | Endoplasmic reticulum aminopeptidase 2 (Leukocyte-derived arginine aminopeptidase [L-RAP]) Isoforms 1 to 4_Q6P179−1 to −4; Normal tissues weak to moderate staining. Alveolar macrophages strong. Salivary gland, pancreas, cells in CNS and parathyroid neg. TB & Decidua moderate [15] | 1.00 × 103 pg/mL Plasma [18] | 12_1 |
| FSTL3 -10.0 ↓ | Follistatin-related protein 3 (Follistatin-like protein 3,Follistatin-related gene protein Isoforms 1 to 2)_O95633−1 to −2; Most normal tissues moderate to strong. TB moderate/Decidua strong [15] | 1.20 × 104 pg/mL Plasma [18]; 1.37–1.70 ng/mL 11–15 weeks [26]; 7013–55,484 pg/mL 1st trimester [27] | 3_3 |
| IGFBP1 -10.3 ↓ | Insulin-like growth factor-binding protein 1_P08833; Majority of normal tissues neg. Moderate to strong in subset of basal cells in testis. TB weak/Decidua moderate to strong [15] | 6.00 × 104 pg/mL Plasma [18]; 24–83 ng/mL [28]; 1st and 2nd trimester; 9.34 ± 1.34 mg/L nonpregnant [29] | 40_7 |
| C6orf142; MLIP -10.7 ↓ | Muscular LMNA-interacting protein_Q5VWP3; TB neg/Decidua not available [15] | - | - |
| SART3 -13.8 ↓ | Squamous cell carcinoma antigen recognized by T-cells 3 ([SART-3, hSART-3] Tat-interacting protein of 110 kDa [Tip110] Isoform 3)_Q15020-1; B-lymphocyte derived cell lines, fibroblast cell lines, B lymphoblastoid cell lines, brain, lymphoblastoid cell lines, PBMCs, colon, embryonic cell lines, epithelial cell lines, heart, kidney cell lines, liver cells, lung, pancreatic tissue, peripheral blood leukocytes, placenta, plasma, small intestine, spleen, testicular tissue, thymus gland [19]. TB & Decidua strong [15] | Plasma [18] | 15_0 |
| Transcribed locus AFFY ID 242842_AT 14.5 ↓ | Gamma-Parvin_Q9HBI0; Mainly stained moderately in glandular and transitional epithelial tissues. Lymph node moderate, hematopetic cells weak. TB weak/Decidua neg [15] | - | 12_1 |
| EPAS1 -15.3 ↓ | Endothelial PAS domain-containing protein 1_Q99814; Most normal tissues moderate positivity. Strong in thyroid gland. Distal tubules stronger staining than proximal tubules. TB moderate/Decidua neg [15] | Plasma [18] | 13_0 |
| PAEP -15.6 ↓ | Glycodelin ([GD], Placental protein 14 [PP14], Pregnancy-associated endometrial alpha-2 globulin [PAEG, PEG], Progestagen-associated endometrial protein, Progesterone-associated endometrial protein, Isoform 1 to 3)_P09466−1 to −3; Endometrial glands, placenta and fallopian tube distinct expression. TB moderate/Decidua neg [15] | 116–870 ng/mL 1st, 30–228 ng/mL 2nd, 13–74 ng/mL 3rd trimesters [30]; 17–497 uIU/L nonpregnant [31] | 17_1 |
| MMP12 -17.2 ↓ | Macrophage metalloelastase ([MME], Macrophage elastase [ME, hME], Matrix metalloproteinase-12 [MMP-12])_P39900; Most normal tissues weak to moderate. Seminal vesicle strong. Squamous epithelia, breast glands and neuronal tissues generally neg. TB moderate/Decidua neg [15] | Macrophages, basophils [19] | 14_1 |
| LAIR2 -18.9 ↓ | Leukocyte-associated immunoglobulin-like receptor 2 ([LAIR-2], CD antigen CD306, Isoforms 1 and 2)_Q6ISS4-1; Synovial fluid, urine, B-lymphocyte derived cell lines, PBMCs [19]. Pending THPA [15] | 1–2 mg/mL plasma 1st trimester (Our unpublished data) | 2_1 |
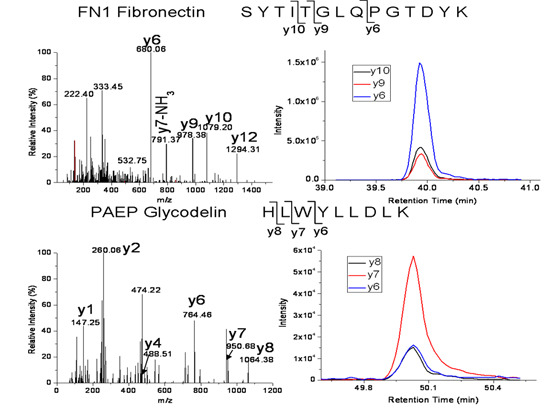
2.1.2. MRM Assay Development

| Gene | Protein | MRM Assay | Mean MRM Peak Area a | |||
|---|---|---|---|---|---|---|
| Peptide Sequence | Transitions | Crude Serum | Hu14 Depleted Serum | Hu6 Depleted Serum | ||
| BTD | Biotinidase | VDLITFDTPFAGR | 726.4 → 1011.5, 910.4, 648.3 | 1.10 × 106 | 1.11 × 107 | 3.05 × 106 |
| LUM | Lumican | LPSGLPVSLLTLYLDNK | 979.1 → 1489.8, 1293.7, 766.4 | 2.57 × 105 | 6.35 × 106 | 1.20 × 106 |
| MBL2 | Mannose-binding protein C | TEGQFVDLTGNR | 668.8 → 1106.6, 921.5, 774.4 | 2.13 × 105 | 4.80 × 106 | 1.19 × 106 |
| CADH5 | Cadherin-5 | EYFAIDNSGR | 586.3 → 879.4, 732.4, 548.2 | 2.14 × 105 | 2.51 × 106 | 8.62 × 105 |
| S100A8 | Protein S100-A8 | LLETECPQYIR | 711.4 → 1195.5, 1066.5, 836.4 | 1.86 × 105 | 1.66 × 106 | 5.42 × 105 |
| CRP | C-reactive protein | GYSIFSYATK | 568.8 → 916.5, 829.4, 716.4 | 3.92 × 105 | 8.91 × 105 | 3.98 × 105 |
| Gene Name | Protein Name | Peptide Sequence | Transitions |
|---|---|---|---|
| FN1 | Fibronectin isoform 1 | SYTITGLQPGTDYK | 772.4 → 680.3, 978.5, 1079.5 |
| FN1 | Fibronectin isoform 1 | EESPLLIGQQSTVSDVPR | 978.0 → 672.4, 860.4, 1173.6 |
| PAEP | Glycodelin | HLWYLLDLK | 600.8 → 764.4, 950.5, 1063.6 |
| CFHR1 | Complement factor H-related protein 1 | ITCTEEGWSPTPK | 753.4 → 529.3, 772.4, 901.4, 1131.5 |
| S100A8 | Protein S100-A8 | LLETECPQYIR | 711.4 → 836.4, 965.4, 1066.5, 1195.5 |


2.1.3. Support for the Developed MRM Assays
| Group (n = 41) | Case (5) | Control (36) | p-Value |
|---|---|---|---|
| Parity (n > 0) | 0 | 1 | 0.12 |
| Gestational age at sampling (mean, SD) | 9.7 (1.2) | 11.1 (0.8) | 0.002 * |
| Race (n not Caucasian) | 0 | 7 | 1.00 |
| BMI (mean, SD) | 27.4 (7.0) | 25.4 (5.8) | 0.50 |
| Smoker in pregnancy (n) | 0 | 6 | 1.00 |
| Average systolic blood pressure in labor (mean, SD) | 145.4 (20.5) | 119.9 (8.5) | 0.0 * |
| Average diastolic blood pressure in labor (mean, SD) | 89.4 (8.6) | 71.4 (10.5) | 0.001 * |
| Gestational age at delivery (mean, SD) | 39.0 (1.2) | 39.6 (1.6) | 0.49 |
| Birthweight (mean, SD) | 2928.2 (491.6) | 3357.7 (545.1) | 0.10 |
| Less than 10th percentile (n with SGA) | 3 | 6 | 0.06 |
2.1.4. Application of MRM Assays to Case and Control Samples
| Gene Name | Accession & Peptides | Mean (± SD) | p-Value (Rank Sum) | |
|---|---|---|---|---|
| Case | Control | |||
| FN1 | EESPLLIGQQSTVSDVPR | 1.26 × 107 (±5.65 × 106) | 1.29 × 107 (±5.78 × 106) | 0.78 |
| FN1 | SYTITGLQPGTDYK | 4.02 × 107 (±1.55 × 107) | 4.41 × 107 (±2.10 × 107) | 0.34 |
| PAEP | HLWYLLDLK | 2.95 × 106 (±1.45 × 106) | 2.57 × 106 (±1.35 × 106) | 0.74 |
| CFHR1 | ITCTEEGWSPTPK | 1.01 × 107 (±4.09 × 106) | 1.27 × 107 (±6.59 × 106) | 0.54 |
| S100A8 | LLETECPQYIR | 2.73 × 106 (±1.50 × 106) | 2.96 × 106 (±1.44 × 106) | 0.62 |
2.2. ELISAs with Additional Candidates
2.3. Immunoblotting of Candidates with Secreted Protein
| Candidate | Result | Candidate | Result |
|---|---|---|---|
| CCK | Requires RIA | MMP-12 | No signal |
| CFH-1 | No signal | S100-A8 | No signal |
| FN-1 | Signal vs. none | SEMA-3C | No signal |
| LAIR-2 | Signal vs. none | SLC16-A6 | One ab; no rhP * |
3. Discussion
4. Experimental Section
4.1. Samples
4.2. MRM Assays
4.2.1. Database Search
4.2.2. Immunoaffinity Removal of High Abundant Serum Proteins
4.2.3. MRM Assay Development
4.2.4. Validation Studies
4.3. ELISAs with Additional Candidates
4.4. Immunoblotting
| Candidate | Catalog Number, Company; Site | Candidate | Catalog Number, Company; Site |
|---|---|---|---|
| CFH-1 | MAB4779, Clone 556317 mAb & 4779-FH-050 rhCFH-1, R&D Systems, Minneapolis, MN | MMP-12 | MAB919 mAb, AF917 pAb & WBC019 rhMMP-12, R&D Systems |
| FN-1 | ab154211 mAb, Cambridge, MA; #161-0375 rhFN-1, Bio Rad Hercules, CA | S100-A8 | MAB4570, Clone 749916 mAb & 8226-S8-050, rhS100A8/S100A9 heterodimer, R&D Systems |
| LAIR-2 | 1A7/FMU-LAIR-2 mAb gift of Boquan Jin; MAB2665 mAb & 2665-LR-050 rhLAIR-2, R&D Systems | SEMA-3C | MAB1728 Clone 238835 mAb & 5570-S3-050 rhSemaphorin 3C Fc Chimera; R&D Systems |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghulmiyyah, L.; Sibai, B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 2012, 36, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Benjamin, E.J.; Berra, K. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the American Heart Association. Circulation 2011, 22, 1243–1262. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. Br. Med. J. 2007, 335, 974–977. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Von Dadelszen, P.; Magee, L.A.; Roberts, J.M. Subclassification of preeclampsia. Hypertens. Preg. 2003, 22, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Founds, S.; Catov, J.; Gallaher, M.; Harger, G.; Markovic, N.; Roberts, J. Is there evidence of separate inflammatory or metabolic forms of preeclampsia. Hypertens. Preg. 2011, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Founds, S.; Conley, Y.P.; Lyons-Weiler, J.F.; Jeyabalan, A.; Hogge, W.A.; Conrad, K.P. Global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta 2009, 301, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, A.; Brown, M.; Zeeman, G.; Deeker, G.; Sibai, B. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Preg. Hypertens. 2013, 3, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.; de Wolf, F.; Robertson, W.B.; Brosens, I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br. J. Obstet. Gynaecol. 1986, 93, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Meekins, J.; Pijnenborg, R.; Hanssens, M.; McFadyen, I.R.; van Asshe, A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br. J. Obstet. Gynaecol. 1994, 101, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Founds, S.A.; Terhorst, L.A.; Conrad, K.P.; Hogge, W.A.; Jeyabalan, A.; Conley, Y.P. Gene expression of eight candidates in first trimester preeclampsia placenta. Biol. Res. Nurs. 2011, 132, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Founds, S.A. Bridging global gene expression candidates in first trimester placentas with susceptibility loci from linkage studies of preeclampsia. J. Perinat. Med. 2011, 39, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Founds, S.A.; Shi, H.; Conley, Y.P.; Jeyabalan, A.; Roberts, J.M.; Lyons-Weiler, J. Variations in discovery-based preeclampsia candidate genes. Clin. Transl. Sci. 2012, 5, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Prot. 2002, 1, 845–867. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M. Proteomics: Tissue-based map of the human proteome. Science 2015, 23, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Sodowski, K.; Zwirska-Korczala, K.; Kuka, D.; Kukla, M.; Budziszewska, P.; Czuba, B.; Włoch, A.; Cnota, W.; Bielański, W.; Brzozowski, T.; et al. Basal and postprandial gut peptides affecting food intake in lean and obese pregnant women. J. Physiol. Pharmacol. 2007, 58, 37–52. [Google Scholar] [PubMed]
- Frick, G.; Bremme, K.; Sjögren, C.; Lindén, A.; Uvnäs-Moberg, K. Plasma levels of cholecystokinin and gastrin during the menstrual cycle and pregnancy. Acta Obstet. Gynecol. Scand. 1990, 69, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S. Data management and data integration in the HUPO plasma proteome project. Meth Mol. Biol. 2011, 696, 247–257. [Google Scholar]
- Ingenuity Pathways Analysis (IPA®, QIAGEN, Redwood City, CA, USA). Available online: http://www.qiagen.com/ingenuity (accessed on 13 June 2015).
- Islami, D.; Shoukir, Y.; Dupont, P.; Campana, A.; Bischof, P. Is cellular fibronectin a biological marker for pre-eclampsia? Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 97, 40–45. [Google Scholar] [CrossRef]
- Powers, R.W.; Catov, J.M.; Bodnar, L.M.; Gallaher, M.J.; Lain, K.Y.; Roberts, J.M. Evidence of endothelial dysfunction in preeclampsia and risk of adverse pregnancy outcome. Reprod. Sci. 2008, 15, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Sobocka, M.B.; Sobocki, T.; Banerjee, P.; Weiss, C.; Rushbrook, J.I.; Norin, A.J.; Hartwig, J.; Salifu, M.O.; Markell, M.S.; Babinska, A.; et al. Cloning of the human platelet F11 receptor: A cell adhesion molecule member of the immunoglobulin superfamily involved in platelet aggregation. Blood 2000, 95, 2600–2609. [Google Scholar] [PubMed]
- Ostermann, G.; Weber, K.S.; Zernecke, A.; Schroder, A.; Weber, C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 2002, 3, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Leung, R.Y.; Babinska, A.; Salifu, M.O.; Ehrlich, Y.H.; Kornecki, E.; Wong, L.Y.; Tso, A.W.; Cherny, S.S.; Sham, P.C.; et al. Elevated plasma level of soluble F11 receptor/junctional adhesion molecule-A (F11R/JAM-A) in hypertension. Am. J. Hypertens. 2009, 22, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Karadag, A.; Fohr, B.; Fisher, L.W.; Fedarko, N.S. Three SIBLINGs (small integrin-binding ligand, N-linked glycoproteins) enhance factor H’s cofactor activity enabling MCP-like cellular evasion of complement-mediated attack. J. Biol. Chem. 2002, 277, 913700–913708. [Google Scholar] [CrossRef] [PubMed]
- Miron, P.; Lambert, J.; Marcil, A.; Cowans, N.J.; Stamatopoulou, A.; Spencer, K. Maternal plasma levels of follistatin-related gene protein in the first trimester of pregnancies with Down syndrome. Prenat. Diagn. 2010, 30, 224–280. [Google Scholar] [CrossRef] [PubMed]
- Apps, R.; Sharkey, A.; Gardner, L.; Male, V.; Trotter, M.; Miller, N.; North, R.; Founds, S.; Moffett, A. Genome-wide expression profile of first trimester villous and extravillous human trophoblast cells. Placenta 2011, 32, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Vatten, L.J.; Nilsen, T.I.; Juul, A.; Jeansson, S.; Jenum, P.A.; Eskild, A. Changes in circulating level of IGF-I and IGF-binding protein-1 from the first to second trimester as predictors of preeclampsia. Eur. J. Endocrinol. 2008, 158, 101–150. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.M.; Ezzat, S.; Axelrad, A.A. Insulin-like growth factor binding protein-1 is elevated in patients with polycythemia vera and stimulates erythroid burst formation in vitro. Blood 1997, 89, 1862–1869. [Google Scholar] [PubMed]
- Loukovaara, S.; Immonen, I.R.; Loukovaara, M.J.; Koistinen, R.; Kaaja, R.J. Glycodelin: A novel serum anti-inflammatory marker in type 1 diabetic retinopathy during pregnancy. Acta Ophthalmol. Scand. 2007, 85, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, L.G.; Wiberg, N.; Andersen, C.Y.; Laursen, S.B.; Kliem, A.; Westergaard, J.G.; Teisner, B. Circulating concentrations of placenta protein 14 during the natural menstrual cycle in women significantly reflect endometrial receptivity to implantation and pregnancy during successive assisted reproduction cycles. Hum. Reprod. 1998, 13, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Chappell, S.; Morgan, L. Searching for genetic clues to the causes of pre-eclampsia. Clin. Sci. 2006, 110, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Founds, S.A.; Dorman, J.S.; Conley, Y.P. Microarray technology applied to the complex disorder of preeclampsia. J. Obstet. Gynecol. Neonatal Nurs. 2008, 37, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Entrez Gene. Available online: http://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/gene?cmd=Retrieve&dopt=full_report&list_uids=5047 (accessed on 20 June 2015).
- Meyaard, L.; Adema, G.J.; Chang, C.; Woollatt, E.; Sutherland, G.R.; Lanier, L.L.; Phillips, J.H. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 1997, 7, 283–290. [Google Scholar] [CrossRef]
- Lebbink, R.J.; van den Berg, M.C.; de Ruiter, T.; Raynal, N.; van Roon, J.A.; Lenting, P.J.; Jin, B.; Meyaard, L. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR-1 inhibitory immune interaction. J. Immunol. 2008, 180, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Founds, S.A.; Fallert-Junecko, B.; Reinhart, T.A.; Conley, Y.P.; Parks, W.T. LAIR2 localizes specifically to sites of extravillous trophoblast invasion. Placenta 2010, 31, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Founds, S.A.; Fallert-Junecko, B.; Reinhart, T.A.; Parks, W.T. LAIR2-expressing extravillous trophoblasts associate with maternal spiral arterioles undergoing physiologic conversion. Placenta 2013, 34, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Reproducibility crisis: Blame it on the antibodies. Nature 2015, 521, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Bordeaux, J.; Welsh, A.; Agarwal, S.; Killiam, E.; Baquero, M.; Hanna, J.; Anagnostou, V.; Rimm, D. Antibody validation. Biotechniques 2010, 48, 197–209. [Google Scholar] [CrossRef] [PubMed]
- White, E.S.; Baralle, F.E.; Muro, A.F. New insights into form and function of fibronectin splice variants. J. Pathol. 2008, 216, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rasanen, J.; Quinn, M.J.; Laurie, A.; Bean, E.; Roberts, C.T., Jr.; Nagalla, S.R.; Gravett, M.G. Maternal serum glycosylated fibronectin as a point-of-care biomarker for assessment of preeclampsia. Am. J. Obstet. Gyn. 2015, 212, 82.e1–82.e9. [Google Scholar] [CrossRef] [PubMed]
- Rasanen, J.P.; Snyder, C.K.; Rao, P.V.; Mihalache, R.; Heinonen, S.; Gravett, M.G.; Roberts, C.T., Jr.; Nagalla, S.R. Glycosylated fibronectin as a first-trimester biomarker for prediction of gestational diabetes. Obstet. Gynecol. 2013, 122, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.K.; Smith, S.D.; Keogh, R.J.; Jones, R.L.; Baker, P.N.; Knöfler, M.; Cartwriqht, J.E.; Whitley, G.S.; Aplin, J.D. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am. J. Pathol. 2010, 177, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Founds, S.A.; Ren, D.; Roberts, J.M.; Jeyabalan, A.; Powers, R.W. Follistatin-like 3 across gestation in preeclampsia and uncomplicated pregnancies among lean and obese women. Reprod. Sci. 2015, 22, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Sidis, Y.; Tortoriello, D.V.; Holmes, W.E.; Pan, Y.; Keutmann, H.T.; Schneyer, A.L. Follistatin-related protein and follistatin differentially neutralize endogenous vs. exogenous activin. Endocrinology 2002, 143, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Bartholin, L.; Maguer-Satta, V.; Hayette, S.; Martel, S.; Gadoux, M.; Corbo, L.; Magaud, JP.; Rimokh, R. Transcription activation of FLRG and follistatin by activin A, through Smad proteins, participates in a negative feedback loop to modulate activin A function. Oncogene 2002, 21, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, D.; Liao, Q.P.; Yang, H.X.; Cao, B.; Fu, G.; Ye, G.; Bai, Y.; Wang, H.; Cui, N.; et al. High levels of activin A detected in preeclamptic placenta induce trophoblast cell apoptosis by promoting nodal signaling. J. Clin. Endocrinol. Metab. 2012, 97, E1370–E1379. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, R.; Powe, C.E.; Tjoa, M.L.; Khankin, E.; Ye, J.; Ecker, J.; Schneyer, A.; Karumanchi, S.A. First trimester follistatin-like-3 levels in pregnancies complicated by subsequent gestational diabetes mellitus. Diabetes Care 2010, 33, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Pearson, G.; Cutler, J.; Lindheimer, M. NHLBI Working Group. Summary of the NHLBI Working Group on research on hypertension during pregnancy. Hypertens 2003, 41, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.W.; Lam, H.; Aebersold, R. PeptideAtlas: A resource for target selection for emerging targeted proteomics workflows. EMBO Rep. 2008, 9, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hood, B.L.; Zhao, T.; Conrads, T.P.; Sun, M.; Gopalakrishnan, V.; Grover, H.; Day, R.S.; Weissfeld, J.L.; Wilson, D.O.; et al. Lung cancer serum biomarker discovery using label-free liquid chromatography-tandem mass spectrometry. J. Thorac Oncol. 2011, 6, 725–735. [Google Scholar] [CrossRef] [PubMed]
- R&D resources. Available online: https://www.rndsystems.com/resources/technical/western-blot-conditions (accessed on 1 June 2014).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Founds, S.; Zeng, X.; Lykins, D.; Roberts, J.M. Developing Potential Candidates of Preclinical Preeclampsia. Int. J. Mol. Sci. 2015, 16, 27208-27227. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126023
Founds S, Zeng X, Lykins D, Roberts JM. Developing Potential Candidates of Preclinical Preeclampsia. International Journal of Molecular Sciences. 2015; 16(11):27208-27227. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126023
Chicago/Turabian StyleFounds, Sandra, Xuemei Zeng, David Lykins, and James M. Roberts. 2015. "Developing Potential Candidates of Preclinical Preeclampsia" International Journal of Molecular Sciences 16, no. 11: 27208-27227. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms161126023