Localization and Spectroscopic Analysis of the Cu(I) Binding Site in Wheat Metallothionein Ec-1
Abstract
:1. Introduction
2. Results and Discussion
2.1. Addition of One Equivalent of Cu(I) to Zn6Ec-1
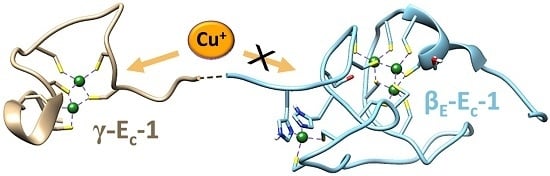
2.2. Identification of the Cu(I) Binding Domain
2.3. Spectroscopic Studies of Cu(I) Binding to the Separate γ-Ec-1 Domain
2.4. Metal Ion to Protein Stoichiometries of γ-Ec-1 Species Formed during the Titrations with Cu(I) Ions
2.5. Biologically Relevant or an Artifact?
3. Materials and Methods
3.1. Chemicals and Solutions
3.2. Protein Expression and Purification
3.3. Proteinase K Cleavage
3.4. Titration of γ-Ec-1 with Cu(I)
3.5. CD Spectroscopy
3.6. Mass Spectrometry
3.7. Luminescence Spectroscopy
3.8. DLS Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2-PDS | 2,2′-dithio-dipyridine | F-AAS | Flame atomic absorption spectroscopy |
| CD | Circular dichroism | LMCT | Ligand-to-metal charge transfer |
| DLS | Dynamic light scattering | MT | Metallothionein |
| ESI-MS | Electrospray ionization mass spectrometry | SEC | Size exclusion chromatography |
References
- Blindauer, C.A.; Leszczyszyn, O.I. Metallothioneins: Unparalleled diversity in structures and functions for metal ion homeostasis and more. Nat. Prod. Rep. 2010, 27, 720–741. [Google Scholar] [CrossRef] [PubMed]
- Vašák, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Maret, W.; Vallee, B.L. Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 3489–3494. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Someya, A.; Komada, Y.; Ogra, Y. Roles of metallothionein in copper homeostasis: Responses to Cu-deficient diets in mice. J. Inorg. Biochem. 2002, 88, 173–182. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.S.; Patel, K.M.; Roesijadi, G. Oyster metallothionein as an oxyradical scavenger: Implications for hemocyte defense responses. Dev. Comp. Immunol. 1999, 23, 443–449. [Google Scholar] [CrossRef]
- Kojima, Y.; Binz, P.-A.; Kägi, J.H.R. Nomenclature of metallothionein: Proposal for a revision. In Metallothionein IV, 1st ed.; Klaassen, C., Ed.; Birkhäuser Verlag: Basel, Switzerland, 1999; pp. 3–6. [Google Scholar]
- Binz, P.-A.; Kägi, J.H.R. Metallothionein: Molecular evolution and classification. In Metallothionein IV; Klaassen, C., Ed.; Birkhäuser Verlag: Basel, Switzerland, 1999; pp. 7–13. [Google Scholar]
- Lane, B.G.; Kajioka, R.; Kennedy, T.D. The wheat-germ Ec protein is a zinc-containing metallothionein. Biochem. Cell Biol. 1987, 65, 1001–1005. [Google Scholar] [CrossRef]
- Kawashima, I.; Kennedy, T.D.; Chino, M.; Lane, B.G. Wheat Ec metallothionein genes. Like mammalian Zn2+ metallothionein genes, wheat Zn2+ metallothionein genes are conspicuously expressed during embryogenesis. Eur. J. Biochem. 1992, 209, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Peroza, E.A.; Al Kaabi, A.; Meyer-Klaucke, W.; Wellenreuther, G.; Freisinger, E. The two distinctive metal ion binding domains of the wheat metallothionein Ec-1. J. Inorg. Biochem. 2009, 103, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Peroza, E.A.; Freisinger, E. Metal ion binding properties of Triticum aestivum Ec-1 metallothionein: Evidence supporting two separate metal-thiolate clusters. J. Biol. Inorg. Chem. 2007, 12, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Loebus, J.; Peroza, E.A.; Blüthgen, N.; Fox, T.; Meyer-Klaucke, W.; Zerbe, O.; Freisinger, E. Protein and metal cluster structure of the wheat metallothionein domain g-Ec-1. The second part of the puzzle. J. Biol. Inorg. Chem. 2011, 16, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Peroza, E.A.; Schmucki, R.; Güntert, P.; Freisinger, E.; Zerbe, O. The bE-domain of the wheat Ec-1 metallothionein: A metal-binding domain with a distinctive structure. J. Mol. Biol. 2009, 387, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Palacios, Ò.; Atrian, S.; Capdevila, M. Zn- and Cu-thioneins: A functional classification for metallothioneins? J. Biol. Inorg. Chem. 2011, 16, 991–1009. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Thomas, M.A.; Thurman, D.A.; Tomsett, A.B. Metallothionein genes from the flowering plant Mimulus guttatus. FEBS Lett. 1990, 260, 277–280. [Google Scholar] [CrossRef]
- Fordham-Skelton, A.P.; Wilson, J.R.; Groom, Q.; Robinson, N.J. Accumulation of metallothionein transcripts in response to iron, copper and zinc: Metallothionein and metal-chelate reductase. Acta Physiol. Plant. 1997, 19, 451–457. [Google Scholar] [CrossRef]
- Van Hoof, N.A.L.M.; Hassinen, V.H.; Hakvoort, H.W.J.; Ballintijn, K.F.; Schat, H.; Verkleij, J.A.C.; Ernst, W.H.O.; Karenlampi, S.O.; Tervahauta, A.I. Enhanced copper tolerance in Silene vulgaris (Moench) Garcke populations from copper mines is associated with increased transcript levels of a 2b-type metallothionein gene. Plant Physiol. 2001, 126, 1519–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.M.; Goldsbrough, P.B. Functional homologs of fungal metallothionein genes from Arabidopsis. Plant Cell 1994, 6, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-J.; Meetam, M.; Goldsbrough, P. Examining the specific contributions of individual arabidopsis metallothioneins to copper distribution and metal tolerance. Plant Physiol. 2008, 146, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Benatti, M.R.; Yookongkaew, N.; Meetam, M.; Guo, W.J.; Punyasuk, N.; AbuQamar, S.; Goldsbrough, P. Metallothionein deficiency impacts copper accumulation and redistribution in leaves and seeds of Arabidopsis. New Phytol. 2014, 202, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Leszczyszyn, O.I.; Schmid, R.; Blindauer, C.A. Toward a property/function relationship for metallothioneins: Histidine coordination and unusual cluster composition in a zinc-metallothionein from plants. Proteins Struct. Funct. Bioinform. 2007, 68, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Onana, P.; Shaw, C.F.; Petering, D.H. Characterization of calf liver Cu, Zn-metallothionein: Naturally variable Cu and Zn stoichiometries. Biochem. J. 1996, 317, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Ihara, Y. The N-terminal portion of growth-inhibitory factor is sufficient for biological activity. J. Biol. Chem. 1995, 270, 3365–3369. [Google Scholar] [PubMed]
- Uchida, Y.; Takio, K.; Titani, K.; Ihara, Y.; Tomonaga, M. The growth inhibitory factor that is deficient in the Alzheimers-disease brain is a 68-amino acid metallothionein-like protein. Neuron 1991, 7, 337–347. [Google Scholar] [CrossRef]
- Chung, R.S.; Howells, C.; Eaton, E.D.; Shabala, L.; Zovo, K.; Palumaa, P.; Sillard, R.; Woodhouse, A.; Bennett, W.R.; Ray, S.; et al. The native copper- and zinc- binding protein metallothionein blocks copper-mediated Ab aggregation and toxicity in rat cortical neurons. PLoS ONE 2010, 5, e12030. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.; Sonois, V.; Delaine, T.; Guilloreau, L.; Gillet, A.; Teissie, J.; Faller, P.; Vasak, M. Metal swap between Zn7-metallothionein-3 and amyloid-β-Cu protects against amyloid-β toxicity. Nature Chem. Biol. 2008, 4, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmorstein, R.; Carey, M.; Ptashne, M.; Harrison, S.C. DNA recognition by Gal4-structure of a protein DNA complex. Nature 1992, 356, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.O.; Jacobsen, J. Reactivity of the thiol-group in human and bovine albumin at pH 3–9, as measured by exchange with 2,2′-dithiodipyridine. Eur. J. Biochem. 1980, 106, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Pountney, D.L.; Schauwecker, I.; Zarn, J.; Vašák, M. Formation of mammalian Cu8-metallothionein in vitro: Evidence for the existence of two Cu(I)4-thiolate clusters. Biochemistry 1994, 33, 9699–9705. [Google Scholar] [CrossRef] [PubMed]
- Braun, W.; Vašák, M.; Robbins, A.H.; Stout, C.D.; Wagner, G.; Kägi, J.H.R.; Wüthrich, K. Comparison of the NMR solution structure and the X-ray crystal-structure of rat metallothionein-2. Proc. Natl. Acad. Sci. USA 1992, 89, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Calderone, V.; Dolderer, B.; Hartmann, H.J.; Echner, H.; Luchinat, C.; Del Bianco, C.; Mangani, S.; Weser, U. The crystal structure of yeast copper thionein: The solution of a long-lasting enigma. Proc. Natl. Acad. Sci. USA 2005, 102, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Roschitzki, B.; Vašák, M. A distinct Cu4-thiolate cluster of human metallothionein-3 is located in the N-terminal domain. J. Biol. Inorg. Chem. 2002, 7, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Pulla Rao, C.; Dorfman, J.R.; Holm, R.H. Synthesis and structural systematics of ethane-1,2-dithiolato complexes. Inorg. Chem. 1986, 25, 428–439. [Google Scholar]
- Dolderer, B.; Echner, H.; Beck, A.; Hartmann, H.J.; Weser, U.; Luchinat, C.; Del Bianco, C. Coordination of three and four Cu(I) to the α- and β-domain of vertebrate Zn-metallothionein-1, respectively, induces significant structural changes. FEBS J. 2007, 274, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Tarasava, K.; Freisinger, E. Investigating the influence of histidine residues on the metal ion binding ability of the wheat metallothionein g-Ec-1 domain. J. Inorg. Biochem. 2015, 153, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.-B.; Watanabe, T.; Matsuoka, H. A novel copper-binding protein with characteristics of a metallothionein from a clinical isolate of Candida albicans. Microbiology 1999, 145, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Toriumi, S.; Saito, T.; Hosokawa, T.; Takahashi, Y.; Numata, T.; Kurasaki, M. Metal binding ability of metallothionein-3 expressed in Escherichia coli. Basic Clin. Pharmacol. 2005, 96, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Lian, C.L.; Shen, Z.G. Proteomic identification of small, copper-responsive proteins in germinating embryos of Oryza sativa. Ann. Bot. 2009, 103, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Buchecker, C.; Sauvage, J.-P. Templated synthesis of interlocked macrocyclic ligands, the catenands. Preparation and characterization of the prototypical bis-30 membered ring system. Tetrahedron 1990, 46, 503–512. [Google Scholar] [CrossRef]







| +1 Cu(I) | +2 Cu(I) | +3 Cu(I) | |
|---|---|---|---|
| ε262 nm,M−1·cm−1 | 11,521 | 26,208 | 25,226 |
| number −S− | 2.6 | 6.0 | 5.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasava, K.; Loebus, J.; Freisinger, E. Localization and Spectroscopic Analysis of the Cu(I) Binding Site in Wheat Metallothionein Ec-1. Int. J. Mol. Sci. 2016, 17, 371. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030371
Tarasava K, Loebus J, Freisinger E. Localization and Spectroscopic Analysis of the Cu(I) Binding Site in Wheat Metallothionein Ec-1. International Journal of Molecular Sciences. 2016; 17(3):371. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030371
Chicago/Turabian StyleTarasava, Katsiaryna, Jens Loebus, and Eva Freisinger. 2016. "Localization and Spectroscopic Analysis of the Cu(I) Binding Site in Wheat Metallothionein Ec-1" International Journal of Molecular Sciences 17, no. 3: 371. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17030371