Ruthenium(III) Complexes of Heterocyclic Tridentate (ONN) Schiff Base: Synthesis, Characterization and its Biological Properties as an Antiradical and Antiproliferative Agent
Abstract
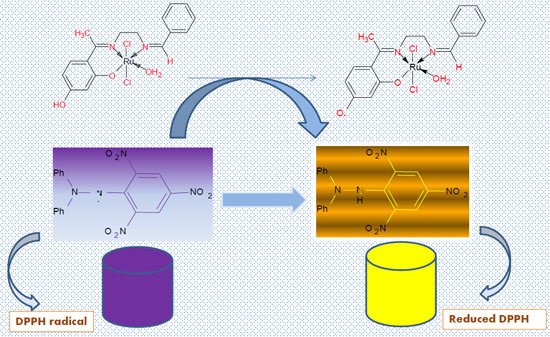
:1. Introduction
2. Results and Discussion
2.1. Synthesis

2.2. Infrared Spectral Studies of the Ru(III) Complexes
2.3. Electronic Absorption Spectra Studies of Heterocyclic Ru(III) Compounds
2.4. The Antioxidant Assay
| Compounds | DPPH Radical Activity | ABTS Radical Activity | ||
|---|---|---|---|---|
| IC50 (µM) | R2 | IC50 (µM) | R2 | |
| [(METBOD)RuCl2] (1) | 2.86 ± 0.57 | 0.971 | 2.98 ± 1.44 | 0.878 |
| [(BZEBOD)RuCl2] (2) | 1.52 ± 0.36 | 0.936 | 3.28 ± 1.26 | 0.967 |
| [(MOABOD)RuCl2] (3) | 1.55 ± 0.54 | 0.973 | 3.29 ± 0.94 | 0.917 |
| [(PAEBOD)RuCl2] (4) | 1.50 ± 0.40 | 0.960 | 3.54 ± 1.31 | 0.812 |
| Vitamin C * | 1.92 ± 1.07 | 0.978 | - | - |
| Rutin * | 2.52 ± 1.60 | 0.798 | 2.83 ± 1.84 | 0.983 |
| BHT * | - | - | 1.64 ± 1.54 | 0.919 |
2.4.1. (DPPH) Free Radical Scavenging Activity (FRSA) Assay

2.4.2. ABTS Scavenging Property of Heterocyclic Ru(III) Compounds
| Anticancer Activity IC50 (µM) 48 h | ||||
|---|---|---|---|---|
| Compounds | Molecular Formula | TK-10 | UACC-62 | MCF-7 |
| [(BZEBOD)RuCl2] (2) | C17H21N2O4RuCl2 | 10.34 ± 1.35 | 6.63 ± 1.92 | 3.63 ± 1.92 |
| [(MOABOD)RuCl2] (3) | C18H23N2O5RuCl2 | 14.47 ± 0.98 | 6.27 ± 0.89 | 3.99 ± 1.45 |
| [(PAEBOD)RuCl2] (4) | C18H23N2O4RuCl2 | 11.85 ± 4.50 | 4.88 ± 0.53 | 3.79 ± 3.03 |
| Parthenolide * (D) | C15H20O3 | 0.50 ± 1.43 | 0.89 ± 2.18 | 0.44 ± 2.02 |

2.5. Anti-Proliferative Activity Evaluation

3. Experimental Section
3.1. Materials and Methods
3.2. Synthetic Procedure for Heterocycles (METBOD, BZEBOD, MOABOD, PAEBOD)
3.3. General Procedure for the Synthesis of Ru(III) Compounds
3.4. The Antioxidant Assay
3.4.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity (FRSA) Assay
3.4.2. ABTS Radical Scavenging Prospects
3.5. Cell Viability Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vigato, P.A.; Tamburini, S.; Bertols, L. The development of compartmental macrocyclic Schiff bases and related polyamine derivatives. Coord. Chem. Rev. 2007, 251, 1311–1492. [Google Scholar] [CrossRef]
- De Geest, D.J.; Noble, A.; Moubaraki, B.; Murray, K.S.; Larsen, D.S.; Brooker, S. Dicopper(II) complexes of a new pyrazolate-containing Schiff base macrocycle and related acyclic ligand. Dalton Trans. 2007, 28, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Abdalrazaq, E.A.; Al-Ramadane, O.M.; Al-Numa, K.S. Synthesis and characterization of dinuclear metal complexes stabilized by tetradentate Schiff base ligands. Am. J. Appl. Sci. 2010, 7, 628–633. [Google Scholar] [CrossRef]
- Agarwal, B.V.; Hingorani, S. Characteristics IR and electronic spectral studies on novel mixed ligand complexes of copper(II) with thiosemicarbazones and heterocyclic bases. Synth. React. Inorg. Met-Org. Chem. 1990, 20, 123–132. [Google Scholar]
- Mittal, P.; Joshi, S.; Panwar, V.; Uma, V. Biologically active Co(II), Ni(II), Cu(II) and Mn(II) complexes of Schiff bases derived from vinyl aniline and heterocyclic aldehydes. Int. J. ChemTech Res. 2009, 1, 225–232. [Google Scholar]
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, V.G.; Nair, B.U. Synthesis, characterization and electrochemical studies of mixed ligand complexes of ruthenium(II) with DNA. Dalton Trans. 2005, 17, 2842–2848. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, P.A.; Aldrich-Wright, J.R. Evidence for chiral discrimination of Ruthenium(II) polypyridyl complexes by DNA. Dalton Trans. 2003, 176–183. [Google Scholar] [CrossRef]
- Maheswari, P.U.; Palaniandavar, M. DNA binding and cleavage activity of [Ru(NH3)4(diimine)]Cl2 complexes. Inorg. Chim. Acta 2004, 357, 901–912. [Google Scholar] [CrossRef]
- Erkkila, K.E.; Odom, D.T.; Barton, J.K. Recognition and reaction of metallointercalators with DNA. Chem. Rev. 1999, 99, 2777–2795. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.-N.; Zou, X.-H.; Liu, J.-G. Shape- and enantioselective interaction of Ru(II)/Co(III) polypyridyl complexes with DNA. Coord. Chem. Rev. 2001, 216, 513–536. [Google Scholar] [CrossRef]
- Gulcin, I.; Oktay, M.; Kirecci, E.; Kufrevioglu, I.O. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Gocer, H.; Menzek, A.; Gulcin, I. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl) methanone and its derivatives. Arch. Pharm. 2012, 345, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, characterization, antioxidant, and antibacterial studies of some metal(ii) complexes of tetradentate Schiff base ligand: (4E)-4-[(2-{(E)-[1-(2,4-Dihydroxyphenyl)ethylidene]amino}ethyl)imino] pentan-2-one. Bioinorg. Chem. Appl. 2015, 2015, 890734. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Pothiraj, K.; Baskaran, T. DNA-binding, oxidative DNA cleavage and coordination mode of later 3d transition metal complexes of a Schiff base derived from isatin as antimicrobial agents. J. Coord. Chem. 2011, 64, 3900–3917. [Google Scholar] [CrossRef]
- Puckett, C.A.; Barton, J.K. Methods to explore cellular uptake of ruthenium complexes. J. Am. Chem. Soc. 2007, 129, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.L.; Che, C.M.; Siu, F.M.; Yang, M.; Wong, K.Y. DNA binding and cytotoxicity of ruthenium(II) and rhenium(I) complexes of 2-amino-4-phenylamino-6-(2-pyridyl)-1,3,5-triazine. Inorg. Chem. 2007, 46, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Pascu, G.I.; Hotze, A.C.G.; Sanchez-Cano, C.; Kariuki, B.M.; Hannon, M.J. Dinuclear Ruthenium(II) triple-stranded helicates: Luminescent supramolecular cylinders that bind and coil DNA and exhibit activity against cancer cell lines. Angew. Chem. 2007, 119, 4452–4456. [Google Scholar] [CrossRef]
- Lincoln, P.; Norden, B. Compounds used in anti-cancer treatment such as: the binuclear DNA threading transition metal complexes and pharmaceutical compositions. Int. Patent WO 99/15535; Int. Appl. PCT/SE98/01655,, 2 November 1999. [Google Scholar]
- Venkatachalam, G.; Ramesh, R. Catalytic and biological activities of Ru(III) mixed ligand complexes containing N,O donor of 2-hydroxy-1-naphthylideneimines. Spectrochim. Acta Part A 2005, 61, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, characterization, in vitro antioxidant and anticancer studies of ruthenium(III) complexes of symmetric and asymmetric tetradentate Schiff bases. J. Coord. Chem. 2015, 68, 2552–2564. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, characterization and biological studies of metal(II) complexes of (3E)-3-[(2-{(E)-[1-(2,4-dihydroxyphenyl)ethylidene]amino}ethyl)imino]-1-phenylbutan-1-one Schiff base. Molecules 2015, 20, 9788–9802. [Google Scholar] [CrossRef] [PubMed]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Casas, J.S.; Alfonso Castineiras, A.; Condori, F.; Couce, M.D.; Russo, U.; Sanchez, A.; Seoane, R.; Sordo, J.; Varela, J.M. Diorganotin(IV)-promoted deamination of amino acids by pyridoxal: SnR22+ complexes of pyridoxal 5′-phosphate and of the Schiff base pyridoxal-pyridoxamine (PLPM), and antibacterial activities of PLPM and [SnR2(PLPM-2H)] (R = Me, Et, Bu, Ph). Polyhedron 2003, 22, 53–65. [Google Scholar] [CrossRef]
- Ali, S.A.; Soliman, A.A.; Aboaly, M.M.; Ramadan, R.M. Chromium, molybdenum and ruthenium complexes of 2-hydroxyacetophenone Schiff bases. J. Coord. Chem. 2002, 55, 1161–1170. [Google Scholar] [CrossRef]
- Kumar, K.N.; Ramesh, R.; Liu, Y. Synthesis, structure and catalytic activity of cycloruthenated carbonyl complexes containing arylazo phenolate ligands. J. Mol. Catal. A: Chem. 2007, 265, 218–226. [Google Scholar] [CrossRef]
- Shelke, V.A.; Jadhav, S.M.; Patharkar, V.R.; Shankarwar, S.G.; Munde, A.S.; Chondhekar, T.K. Synthesis, spectroscopic characterization and thermal studies of some rare earth metal complexes of unsymmetrical tetradentate Schiff base ligand. Arabian J. Chem. 2012, 5, 501–507. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds; Wiley and Sons: New York, NY, USA, 1978. [Google Scholar]
- Thangadurai, T.D.; Ihm, S.K. Novel bidentate ruthenium(III) Schiff base complexes: Synthetic, spectral, electrochemical, catalytic and antimicrobial studies. Transit. Metal Chem. 2004, 29, 189–195. [Google Scholar] [CrossRef]
- Ballhausen, C.J. Introduction to Ligand Field Theory; McGarw Hill: New York, NY, USA, 1962. [Google Scholar]
- Priya, N.P.; Arunachalam, S.; Manimaran, A.; Muthupriya, D.; Jayabalakrishnan, C. Mononuclear Ru(III) Schiff base complexes: Synthesis, spectral, redox, catalytic and biological activity studies. Spectrochim. Acta Part A 2009, 72, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Raju, V.V.; Balasubramanian, K.P.; Jayabalakrishnan, C.; Chinnusamy, V. Synthesis, characterization, antimicrobial activities and DNA–Binding studies of some Ru(III) complexes of Schiff bases. IJABPT 2012, 3, 76–87. [Google Scholar]
- Viuda-Martos, M.; Navajas, Y.R.; Zapata, E.S.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. An Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Liu, J.-W.; Cai, M.-X.; Xin, Y.; Wu, Q.-S.; Ma, J.; Yang, P.; Xie, H.-Y.; Huang, D.-S. Parthenolide induces proliferation inhibition and apoptosis of pancreatic cancer cells in vitro. J. Exp. Clin. Cancer Res. 2010, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, A.; Saikali, M.; Rau, T.; Gali-Muhtasib, H.; Schneider-Stock, R.; Darwiche, N. Inhibition of tumor promotion by parthenolide: Epigenetic modulation of p21. Cancer Prev. Res. 2012, 5, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Gust, R.; Ott, I.; Posselt, D.; Sommer, K. Development of cobalt(3,4-diarylsalen) complexes as tumor therapeutics. J. Med. Chem. 2004, 47, 5837–5846. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.I.; Hussain, I.; Das, H.K.; Mandal, S.S. Overexpression of human histone methylase MLL1 upon exposure to a food contaminant mycotoxin, deoxynivalenol. FEBS J. 2009, 276, 3299–3307. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejidike, I.P.; Ajibade, P.A. Ruthenium(III) Complexes of Heterocyclic Tridentate (ONN) Schiff Base: Synthesis, Characterization and its Biological Properties as an Antiradical and Antiproliferative Agent. Int. J. Mol. Sci. 2016, 17, 60. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010060
Ejidike IP, Ajibade PA. Ruthenium(III) Complexes of Heterocyclic Tridentate (ONN) Schiff Base: Synthesis, Characterization and its Biological Properties as an Antiradical and Antiproliferative Agent. International Journal of Molecular Sciences. 2016; 17(1):60. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010060
Chicago/Turabian StyleEjidike, Ikechukwu P., and Peter A. Ajibade. 2016. "Ruthenium(III) Complexes of Heterocyclic Tridentate (ONN) Schiff Base: Synthesis, Characterization and its Biological Properties as an Antiradical and Antiproliferative Agent" International Journal of Molecular Sciences 17, no. 1: 60. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17010060